Measuring the impossible: X-ray photoelectron spectroscopy of hydrogen and helium
The Science
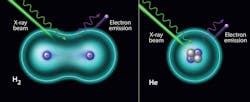
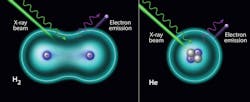
For the first time, and contrary to popular belief, scientists measured the vibrational structure of hydrogen and helium atoms by X-rays.
The Impact
Summary
X-ray photoelectron spectroscopy (XPS) is one of the most powerful techniques in materials science. However, the literature is filled with claims stating that it’s impossible to use XPS to study the two lightest and most abundant elements in the universe, hydrogen and helium. This work demonstrated that ambient pressure X-ray photoelectron spectra of hydrogen and helium can be obtained when a bright-enough X-ray source is used, such as at the National Synchrotron Light Source II. In the case of helium gas, the spectrum shows a symmetric peak from its only orbital. In the case of hydrogen gas molecules, an asymmetric peak is observed, which is related to the different possible vibrational modes of the final state. The hydrogen molecule vibrational structure is evident in the H2 1s spectrum.
Funding
The research carried out in part at the Center for Functional Nanomaterials and the CSX-2 beamline of the National Synchrotron Light Source II, Brookhaven National Laboratory, was supported by the Department of Energy (DOE), Office of Science, Basic Energy Sciences. J.Q. Zhong and M. Wang were supported by a Laboratory Directed Research and Development Project at Brookhaven National Laboratory, and W.H. Hoffmann was supported by the DOE Science Undergraduate Laboratory Internships. This work was supported as part of the Integrated Mesoscale Architectures for Sustainable Catalysis, an Energy Frontier Research Center funded by DOE, Office of Science, Basic Energy Sciences. This research used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science user facility supported by the DOE Office of Science.