Medical Device Susceptibility to RFID Interference
A protocol jointly developed by MET Labs and the FDA has become the first procedure used to test medical devices for interference from radio frequency identification (RFID) signals. Previously, there had been no objective and repeatable way to prove beyond doubt the effect of interference. Now, industry and consumer concerns about the possibilities of RFID devices causing malfunctions in medical equipment can be addressed using scientific methods and techniques.
Until recently, medical equipment manufacturers, RFID vendors, and integrators ignored this topic. However, the concerns grew with every implementation where RFID devices needed to coexist with medical equipment. This could occur by design in hospital rooms or by accident. For example, a customer with a wearable medical device walks into the range of an RFID reader at a retail store or drives by a toll booth. In both intended and accidental exposure cases, patient safety is at stake.
In case of a device malfunction resulting in injury or death, everyone loses, including RFID and medical device manufacturers, hospitals, and integrators. No one has been injured or killed yet, but the situation is a ticking time bomb that is bound to explode some day—and the potential for liability is huge. If this happens, and there is no industry-adopted solution, government regulations may be proposed that might put unnecessary financial burdens on manufacturers.
Until now, medical devices have been subjected to only electromagnetic compatibility (EMC) tests, conducted as part of IEC 60601 standards. These tests are useful, but they do not address RFID.
The potential for RF to interfere with the electronics of medical devices has been identified by several studies. Unfortunately, these studies had several problems including hidden agendas, questionable test approaches, and results that were quoted out of context. Despite these shortcomings, they increased awareness about the interference problem.
In response, the industry has taken steps to make sure that RFID can safely coexist with medical devices. AIM Global’s RFID Experts Group (REG) has established a Healthcare Initiative (HCI) working group to solve the problem. Members of the HCI include many RFID vendors, the FDA, an independent test lab (MET Labs), and several universities.
Standardized Test Methods
The industry solution is to develop standardized test methods that prove beyond any doubt that a medical device can work in the presence of RFID devices emitting all major RFID frequency waves. With these test methods, manufacturers of medical equipment can assess potential RF interference and mitigate it before the devices are on the market.
The test methods cover all equipment that is tested for IEC 60601-1-x and that can be found in the operating room, by a patient’s bedside, or in a doctor’s office: for example, pacemakers, heart pumps, ICDs, defibrillators, artificial pancreases, heart monitors, and glucose monitors.
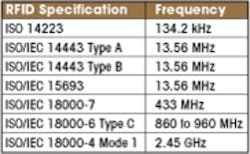
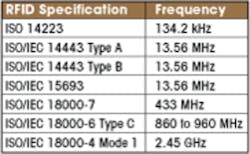
The initial test frequencies in the table include the most common applications of RFID. Other frequencies used by Wi-Fi or WiGig will be added later.
Test Methodology
The basic scientific principle behind the test method is to identify parameters that can cause large bandwidths in the RF signal emitted from the RFID reader (the interrogator). Larger bandwidths correspond to shorter duration pulses that are known to cause interference to digital devices.
MET Labs, together with the FDA, has conducted extensive research to identify parameters and conditions associated with the highest probability of interference for the most commonly used RFID frequency bands in the market today. From the physics perspective, the combination of these parameters is the worst-case scenario in terms of interference.
These parameters form a backbone of the methods that have been developed to test medical devices. In addition, other variables have been integrated, including the distance from the RFID device to a piece of medical equipment.
Test Instruments
At the beginning of the research period, MET Labs received many samples of RFID readers supporting different frequencies. The approach of testing all the readers was very time-consuming and not scalable.
MET then switched to National Instruments’ test platform. The NI platform could emulate readers at all major frequencies. It offered more flexibility because all parameters could be easily set, tested, and evaluated. Furthermore, the tool provided great diagnostic capabilities.
Test Environment
The research and testing were conducted in an anechoic chamber. The chamber ensured there was no outside radio interference; therefore, it was a known and repeatable environment. The same test environment is used for conducting susceptibility tests with real medical equipment.
Next Steps
The next and most important step is to prove that these test methods work in practice. The first device tested by MET Labs was a blood infusion pump. It repeatedly malfunctioned when tested using the new test methods in the presence of UHF signals. In response, the manufacturer’s engineers present at testing developed a solution that involved a protective shield. This quick and inexpensive fix enables the device to function properly in the presence of RFID.
Several other device makers also are preparing to submit their devices for testing. This shows that industry is interested in developing a lasting solution to avoid or mitigate the effect of RFID interference on medical devices. Once RFID readers have been certified for compliance and medical equipment tested for susceptibility, patients can rest assured that the technology around them will help and not hurt them.
To continue refining and validating the test methods, MET Labs is seeking vendors of medical devices covered by the IEC 60601 family of standards to submit their products for testing. As part of the testing, MET will provide free consultation on how to mitigate or eliminate the interference. The identities of the participants and device test results will not be released by MET Labs because the testing is completely confidential. Hospitals also are encouraged to submit their devices for testing.
In addition to test methods, a set of guidelines for the proper operation of RF in real-world applications will be developed to guide RFID implementers. Knowing what combination of parameters to avoid will be of major importance to integrators, hospitals, and other users.
Final Test Protocol
Upon completion and acceptance by AIM, the final test protocol will be submitted to recognized national and international standards organizations so that it may be made publicly available. The end result of this study will benefit all patients, hospitals, medical device manufacturers, and RFID vendors. It will prove that RFID and medical devices can coexist.
About the Author
Ted Osinski is the director of emerging technologies at MET Laboratories and has 25 years of experience in system development, standards organizations, and certification programs. Osinski participates on several standards organizations including EPCglobal and IEEE and consortia such as AIM Global, DASH7, OmniAir, and SmartGrid. He is a past member of W3C Advisory Group, a current member of AIM global RFID Expert Group, and the chairman of the board of OmniAir Certification Services. Osinski earned a M.B.A. from the Farleigh Dickinson University and a B.S. in computer studies from the University of Glamorgan. [email protected]