Research being conducted by Dr. Carson Meredith, Associate Professor at Georgia Tech’s Department of Chemical and Biomolecular Engineering, will directly contribute to the development of low-cost, reliable polymer electrolyte membrane (PEM) fuel cells. While PEM fuel cells have many potential benefits, their high cost and limited durability are slowing the development of potential applications. The most significant application will be serving as the power plant for electric drive trains in hydrogen-powered vehicles.
The basic structure of a fuel cell is an assembly of layers comprised of an electrode, a gas diffusion layer, a catalyst layer, and then the polymer-electrolyte membrane (PEM), followed by the reverse sequence of the same components: catalyst layer, gas-diffusion layer and electrode. At present, Nafion supplied by Du Pont is the most commonly used material for the membrane. The focus of Dr. Meredith’s research is to find suitable lower-cost alternatives for this part of the fuel cell.
According to Dr. Meredith, a recently developed method of high-throughput screening, which was inspired by methods pioneered by the pharmaceutical industry, is vital to this research. The method allows multiple membrane compositions to be evaluated in a single pass. In this method, various PEM compositions are precisely formed as a gradient on a single continuous membrane. The resulting collection of membrane chemistries is called a Library.
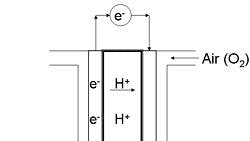
During normal operation, the PEM is hydrated, and the hydrogen-side catalyst causes H2 to separate into 2 electrons and 2 protons. As the electrons from hydrogen flow through the circuit between the fuel cell terminals, the remaining protons of the hydrogen must travel through the water in the membrane, where they will combine with negative oxygen ions on the other side—the rate of proton transport, measured as a conductivity, determines the power output of the cell. This is the primary property screened with Meredith's system.
During a high-throughput conductivity test, the entire library is immersed in 18-MΩ/cm de-ionized, distilled water. The proton conductivity of each membrane composition, for example, at a set of chosen locations, is measured directly by using ac impedance spectroscopy. This is accomplished with a four-point probe that is scanned across the library and is lowered to contact the membrane at specified locations to make a measurement. Each of these measurements takes about 3 seconds, according to Dr. Meredith.
The thickness of the membrane at the measurement sites must be known to calculate conductivity for each composition in the library. Water absorption will affect the thickness of the membrane, which is measured with a spring-loaded micrometer (based on a resistive position sensor) that is also scanned across each library location. The instrument is sensitive to 0.5 microns.
In addition to electrical testing, another high-throughput mechanical screening process has been developed to test the tensile strength of the membrane compositions. In this test, the force required to perforate the membrane is carefully measured in a rapid, point-to-point fashion. When performed after exposing the membrane to operating conditions, mechanical strength serves as an indicator of membrane durability, which is one of the major limitations of today’s membrane technology. A more durable, longer-lasting membrane would equate to lower cost per unit energy. Membranes rupture due to chemical degradation and mechanical stresses, causing fuel crossover, drastic drops in performance, and require replacement of the fuel cell.
A final screening test measures both the permeability and absorption of water in parallel at multiple points on the library. Water is essential to proton transport within the membrane. Today’s membranes dehydrate at temperatures above 80°C, posing a limit to the operating temperature. Operation at higher temperatures, however, would allow the use of less expensive (less pure) hydrogen fuel, such as reformed methanol. As a result, achieving membrane hydration at higher temperatures is another method of making fuel cells more cost effective.
The most difficult aspect of this process of screening is that the membranes must have a combination of several complex properties: ionic conductivity, chemical stability, and water transport and sorption. The strategy behind the use of this screening process is to evaluate the properties of blended materials in order to avoid the more costly synthesis of a single material that has the multiple desired characteristics. The research primarily seeks to minimize the total amount and the unit cost of the material that provides this combination of properties.
This project is sponsored by a grant from the Department of Energy (DOE). Dr. Meredith is presently working with Arkema, the primary grantee and a global chemical company, in the use of this process to evaluate new PEM materials. Recently Dr. Meredith was also awarded a competitive Honda Initiation Grant to continue this research area. The project has already produced positive results, so it’s not too soon for power designers to begin thinking about the best circuit topologies for interfacing familiar loads to PEM fuel cells.