Flexible Batteries Configured as Structural “Skin” Extend Microrobot Runtime
One of the many factors that affects the run time of untethered robots of all sizes is their power/weight ratio. This dilemma is aggravated by the fact that the standard practice of placing the batteries in their own distinct location limited their allowed volume.
In contrast, for most living organisms, motive energy is distributed and stored throughout the body (think muscles); macroscale biological systems don’t have a distinct organ that’s analogous to a discrete battery. Replacing traditional standalone batteries in robotic devices with conformal, multifunctional, biomorphic cells can, in principle, extend their operational time while reducing weight.
Now, a research team based at the University of Michigan (Ann Arbor) is addressing the issue by developing tiny batteries using composites based on aramid nanofibers with a cartilage-like nanoscale structure to combine mechanical and ion transport (battery) properties. The ion-conducting membranes from these composites enable pliable, flexible, zinc-air batteries that can also act as protective covers and support members in robots including soft and flexible miniaturized versions.
The project began with work on the battery materials, chemistry, and charging/discharging cycles using zinc-air battery technology. Nicholas Kotov, the Joseph B. and Florence V. Cejka Professor of Engineering who led the research, acknowledged that “no other structural battery reported is comparable, in terms of energy density, to today’s state-of-the-art advanced lithium batteries.”
Another downside of zinc-air batteries is that they maintain their high capacity for about 100 cycles, rather than the 500 or more that we expect from the lithium-ion batteries in our smartphones. The primary reason for that is the zinc metal forms spikes that eventually pierce the membrane between the electrodes.
Zinc Upside
So why use zinc? The strong yet pliable aramid-nanofiber matrix between the electrodes is the key to the relatively long cycle life for a zinc battery, while the inexpensive and recyclable materials make the batteries easy to replace compared to lithium batteries. Using the zinc-air approach with ion-conducting membranes based on these aramid nanofiber composites (the same carbon-based fibers as found in Kevlar vests) and a water-based polymer gel enable development of pliable batteries that can then be used on the surface of the robotic device.
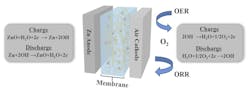
Further, the gel and aramid nanofibers will not catch fire if the battery is damaged, unlike the flammable electrolyte in lithium-ion batteries. The battery materials are abundant and largely nontoxic materials, and the battery is more environmentally friendly than those currently in use. The new battery works by passing hydroxide ions between a zinc electrode and the air side through an electrolyte membrane. That membrane is partly a network of these aramid nanofibers and the gel, which helps transport the hydroxide ions between the electrodes (Fig. 1).
A large part of the team’s initial work involved investigation of different membrane densities and porosities to optimize battery performance while providing extreme flexibility (Fig. 2).
Enter the Robots
The other major part of the project was combining these batteries with the structure of the robots themselves and evaluating their performance with a useful metric of improvement, as the stiffness and pliability of zinc electrodes allows them to be used as conformal load-bearing components. They experimented by fitting the zinc-air batteries on mid-sized and miniature toy robots.
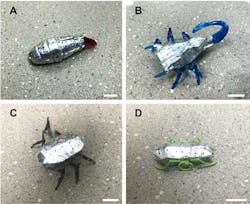
The team replaced their original batteries with zinc-air cells, with the batteries serving as protective covers in both soft and flexible miniaturized robots. They were able to integrate the batteries with soft and flexible elements in the bodies of miniaturized biomorphic robots, including a caterpillar, scorpion, spider, and ant (Fig. 3), despite the mechanical challenges related to the strong deformations and vibrations that are typical in these applications.
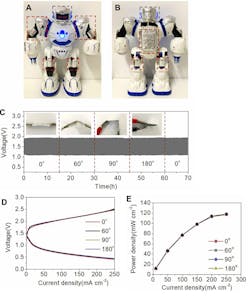
They also used a doll-sized robot toy for evaluation. The stability of the voltage, charge-discharge polarization curves, power density, and ion-conducting properties at different bend angles demonstrated that the battery function and power output didn’t diminish even under stress bending conditions, due to the robust and flexible electrolyte membrane (Fig. 4).
Further, by doing a section-by-section, size-and-space volume analysis for replacing the protective covers of a model robot or supplementing the original power source (Fig. 5), they concluded that the total capacity to be 170 to 340 times higher than that of the current standalone Li-ion battery.
Another conclusion—which frankly seems to be somewhat of an optimistic “stretch” to me—is that their biomorphic batteries could provide 72 times greater capacity and longer operating time (yes, that’s “times” and not “percent”!). “Batteries that can do double duty—to store charge and protect the robot’s ‘organs’—replicate the multifunctionality of fat tissues serving to store energy in living creatures,” said Ahmet Emre, a doctoral student in biomedical engineering in Kotov’s lab.
Their work and tests on battery material, chemistry, and fabrication, along with details of fitting and testing these biomorphic batteries, is thoroughly detailed in their paper “Biomorphic structural batteries for robotics” published in Science Robotics. It’s bolstered by a detailed Supplementary Materials file which also includes links to multiple videos including this interesting one as well as one showing battery removal. This research was funded by the Department of Defense, the National Science Foundation, and the Air Force Office of Scientific Research.