Secure Authentication for Single-Use Medical Consumables
Download this article in PDF format.
Today's medical instruments and their supporting equipment often incorporate single-use consumable sensors, cables, probes, and/or other peripherals. These devices are supplied to the user as sterile products, meaning the device has undergone a process of reducing pathogenic organisms from an object's surface that lead to disease (e.g., viruses, bacterium, fungi).
There are physical, chemical, and radiation methods to sterilize objects for medical applications. The process used is determined through factors such as material compatibility, process availability and location, regulatory approval, speed to market, and cost. For consumables that penetrate or otherwise make contact with a part of the human body, the defined low-sterility-assurance level (SAL—the probability that a single unit that’s subjected to sterilization nevertheless remains nonsterile) is typically at least 10-6, which is a 1 in 1,000,000 chance of a non-sterile unit.
Among the sterilization methods available for high-volume, single-use consumables using gamma radiation from Cobalt-60, a radioisotope which continuously emits gamma-rays, comes with distinct advantages. These include:
- Gamma radiation penetrates deep into irradiated objects.
- The process is faster than chemical methods.
- Processing in gamma irradiators exposes single-use products in their final packaging, so irradiated material can be shipped immediately upon completion of exposure without additional preconditioning.
- It takes place at elevated room temperature and at normal atmospheric pressure.
Sponsored Resources:
- New Memories Breaking the Gamma Barrier for Medical Consumables
- Easily Add Memory, Security, Monitoring, and Control to Medical Sensors and Consumables
- Easily secure your medical disposables
Non-volatile Memory
In many cases, medical consumables can directly benefit from the addition of non-volatile (NV) memory for embedding manufacturing characteristics and operating parameters, data storage, security (to protect against unauthorized aftermarket consumables and to prove OEM authenticity), or to manage limited use or reuse. The added functionality provided by NV memory can also allow for factory calibration of the consumable to the medical instrument.
Up to now, however, these benefits haven’t been realized when gamma irradiation is the required sterilization method for production. This is because gamma radiation is directly incompatible with semiconductor devices that traditionally incorporate floating-gate memory technologies used in NV memories, such as erasable programmable read-only memory (EPROM), electrically erasable programmable read-only memory (EEPROM), and flash memory.
Exposure to gamma's high-ionizing radiation corrupts the logic bit values within these memories, so the relevant data programmed prior to gamma sterilization can’t be retained. Thus, designers have been forced to choose between the added functionality provided by embedded memory and the preferred sterilization method for their products.
Fortunately, user-programmable NV memory ICs now incorporate non-floating-gate technologies in addition to being highly resistant to gamma radiation's high-energy photon bombardment. Gamma-resistant memories such as Maxim Integrated's DS28E80, DS28E83, and DS28E84 will retain their user-programmed data beyond the 20-kGy to 30-kGy (kiloGray, a derived metric [SI] measurement unit of absorbed radiation) dosage levels typically required by the medical industry for sterilization.
The devices communicate over a single-contact 1-Wire bus at standard speed or overdrive speed. The 1-Wire Interface minimizes the Interconnect between a sensor and the instrument. Operating between the host and peripheral over a single pin of the 1-Wire interface reduces interconnect complexity, thus simplifying designs, and lowers cost.
Using these gamma-resistant memories, designers are able to program the embedded memory of their consumables prior to packaging and shipping to a sterilization facility. In addition to gamma-radiation resistance, the memories can incorporate features such as unique factory-programmed identification numbers, user-reprogrammable memory blocks with options of write protection, and, for the Maxim DS28E83 and DS28E84, secure usage management and counterfeit protection
Securing Medical Disposables
The use of disposable tools, sensors, or accessories in the medical field has drastically reduced the amount of healthcare-associated infections. Yet there's still a risk that a disposable device could be reused on a different patient by mistake. What if the medical accessory is designed to be used only a limited number of times and needs to be expired thereafter? And what if the use of a counterfeit accessory causes serious injury to patients or damages the medical equipment?
By incorporating robust protection into a design, technology investments can be successfully defended, brands can be safeguarded, and it helps ensure the trust of patients and healthcare professionals. Maxim offers a portfolio of deep-cover secure authenticators and 1-wire memory devices to protect medical disposables. The parts employ ChipDNA PUF (physically unclonable function) key technology, which is guaranteed unique for every IC.
In PUF-based ChipDNA secure authenticators, each key exists as a precise analog characteristic of the IC, making it immune to invasive attack tools and capabilities: ChipDNA PUF security technology provides an exponential increase in protection against the reverse-engineering attacks that hackers can apply. Attempts to probe or observe ChipDNA operation modifies the underlying circuit characteristics, preventing the discovery of the unique value used by the chip cryptographic functions.
Similarly, more exhaustive reverse-engineering attempts are defeated due to the factory conditioning required to make the ChipDNA circuitry operational. The per-device unique key is generated by the ChipDNA circuitry only when needed for cryptographic operations and then instantaneously deleted.
Most importantly, the ChipDNA secure key never resides statically in registers or memory, nor does it ever leave the electrical boundary of the IC. In addition to the protection benefits, ChipDNA simplifies or eliminates the need for secure IC key management. Maxim’s secure authenticators can be easily integrated into an embedded design without requiring cryptographic expertise. Personalized programming of unique keys is available in the factory.
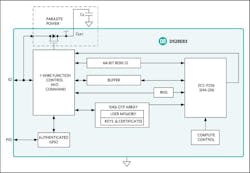
With Maxim Integrated DeepCover authenticators, a secure system can be implemented to protect a product, make sure a disposable isn’t reused, and help patients feel confident that they’re being treated only with authorized products.1. The DS28E83 provides the authentication needed before using a medical instrument. (Source: Maxim)
The 1-Wire DS28E83, for example, provides the authentication and security needed to be certain that only authorized accessories could be used in the system (Fig. 1). By means of this interface, a medical instrument can be authenticated before its use. Or you can securely count the number of times the tool was used—if the number has reached maximum usage, you can discard the tool and the medical instrument at that point will refuse to use it. Similarly, data can be securely updated to the tool. If there’s some calibration data in the medical tool, the instrument can then securely read that, too.
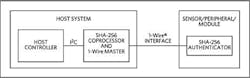
The IC provides a core set of cryptographic tools derived from the integrated asymmetric ECC-P256 and symmetric SHA-256 security functions (Fig. 2). By way of review, public-key cryptography is the science of designing cryptographic systems that employ pairs of keys: a public key that can be distributed freely to anyone, along with a corresponding private key, which is only known to its owner.2. This typical secure authentication system implementation features the DS2465 SHA-256 coprocessor and the DS28E25 SHA-256 authenticator. (Source: Maxim)
Elliptic-curve cryptography (ECC) is an approach to public-key cryptography based on the algebraic structure of elliptic curves over finite fields. ECC requires smaller keys compared to non-EC cryptography. With SHA3-256, the authenticator delivers the latest technology for challenge-and-response for when, say, you’re linking a host system with a sensor/peripheral module. In addition to the security services provided by the hardware-implemented crypto engines, the device integrates a FIPS-compatible true random number generator (TRNG), 10 kb of secured OTP, one configurable GPIO, and a unique 64-bit ROM identification number (ROM ID).
In Summary
Maxim’s DS28E80, DS28E83, and DS28E84 are ICs that employ memory-storage-cell technology that’s highly resistant to the gamma radiation used for sterilization. In addition, the DS28E83 and DS28E84 provide symmetric-key SHA-256 and public-key ECC secure authentication to protect patients against the risks associated with non-qualified counterfeit devices or possible incidental overuse or reuse.
These devices all communicate over the Maxim single-contact 1-Wire bus, with each device having its own guaranteed unique 64-bit serial number that’s factory-programmed into the chip. Taken together—the flexibility of the memory, high radiation resistance, and secure authentication—these devices not only support the memory needs for single-use medical devices, but do so through a single, dedicated contact when the interconnect must be minimized.
Sponsored Resources:
- New Memories Breaking the Gamma Barrier for Medical Consumables
- Easily Add Memory, Security, Monitoring, and Control to Medical Sensors and Consumables
- Easily secure your medical disposables
Related Resources: