Full Material Declarations: Removing Barriers to Environmental Data Reporting
>> Electronic Design Resources
.. >> Library: Article Series
.. .. >> Topic: System Design
.. .. .. >> Series: RoHS and Critical Materials
Download this article in PDF format.
This article was first presented as a paper at the 2018 IPC Apex Expo Technical Conference and published in the 2018 Technical Conference Proceedings. The previous article in this series was “Beyond “Lead-Free: An Update on the IPC-1752A Standard for Materials Declaration.”
Full material declaration of product content in electronics and other industries continues to be a challenge for both suppliers and customers alike. For suppliers, managing substance-level data for all the materials in products is not usually a part of normal business operations, but rather, an added burden and, therefore, cost to doing business. Customers, from mid-supply chain enterprises to OEMs, must have processes and systems to request, manage, and utilize the data to ensure compliance with worldwide substance regulations. These issues call out for an easy-to-use software solution to aid reporting.
The IPC-1752A Materials Declaration Management Standard, which is aligned with IPC-1751A Generic Requirements for Declaration Process Management, is widely used for environmental reporting today. The standard specifies an XML (Extensible Markup Language) schema for mandatory and required data, including support for class D full material declarations (FMDs) for homogenous materials and substances required by the RoHS Directive (the full citation for the current “RoHS Recast” legislation is “Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the restriction of the use of certain hazardous substances in electrical and electronic equipment.”)
In this article, we focus on requirements for tools that enable rapid and accurate reporting of class D FMDs that can be used by suppliers primarily in the base of the supply chain, e.g. raw materials and smaller components. We also provide examples of how this data can be used by the supplier’s immediate customer to build more complex FMD data for product-level assemblies.
Why Take the Road to FMD?
One of the advantages of the FMD approach is that it’s the only way a company can stay ahead of the ongoing addition of regulated substances. RoHS has been relatively static in this regard—with only changes being to allowable Exemptions and additional documentation requirements. Otherwise, the basic six restricted substances have stayed the same from its initial entry into force through its “Recast” in 2011.
The next round of four additional substances, per the European Commissions Delegated Directive 2015/863/EU, will be enforced July 22, 2019. However, customers across the supply chain are already asking for data and compliance conclusions for these substances. This pre-enactment customer-driven activity clearly demonstrates just how valuable FMDs can be since suppliers with FMD data can already satisfy their customer’s requests about the presence of newly (and yet-to-be) restricted substances.
Since RoHS exemptions have set expiration dates, it’s also prudent to know what exempted substance is present, besides just knowing you are Compliant with Exemption, but not exactly why. Since exemptions are substance-specific, this level of information is very useful as a warning that a noncompliance could develop when a product that was once acceptable to ship is no longer compliant because the exemption has expired! FMD data provides the ability to look ahead in time for exemptions that are set to expire, allowing the company to take early action through product redesign or finding alternate suppliers.
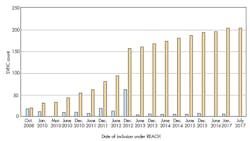
1. Number of REACH SVHCs from 2008 to the present.
REACH (Registration, Evaluation, Authorization and Restriction of Chemicals), promulgated by a separate agency, ECHA (European Chemicals Agency), is much more dynamic and adds new Substances of Very High Concern (SVHC) to the Candidate list roughly twice a year ever since 2008. Figure 1 shows the number of substances added to REACH since its beginning in Oct., 2008 through the last date as of this writing. Shorter gray bars count the substances added each date, with the larger black bars indicating the cumulative total of substances.
Note that there’s some double counting in the Figure 1 data, since a few of the substances were listed a second time due to different toxicological reasons. Also, note that the count is based on just the primary list of SVHC posted by ECHA in its main table, but the actual individual substance count by CAS number is even greater if one consults the ECHA supporting documentation. Further, it’s noted that Amendment 3 of IPC-1752A, which isn’t fully published as of the time of this writing, will contain a non-exhaustive list of substances and their CAS numbers as a convenient reference to this ever-growing list of substances.
Useful information about the Standard and its implementation and advantages may be found on the IPC web page “Data Exchange Standards.” Additional advantages of FMD have been published, for example, by companies offering such services, companies needing FMDs from their suppliers, industry conferences, and articles in electronics journals.1,2 The story continues and the message remains clear: Companies need a way to stay ahead of the growing list of new substances they are required to manage. FMDs are the best way to do so.
Requirements Part One: The Schema is the Roadbed
In the next sections, we list and explain the value of functional requirements that a good, basic FMD reporting tool should have. At the most basic level, the tool by its nature will be software, and to be used for reporting up the supply chain, the XML declaration file must conform to the IPC Standard itself.
In recent years, the chairs and participants in the IPC 2-18b Materials Declaration Task Group have graciously offered their time and expertise to help review, on a blind submission basis, XML files including class D FMDs. Using software tools that are available for checking conformance to the schema as specified in the Standard, as well as a review by IPC 2-18b participants, those software solution providers that have been verified in this review process and found to conform to the schema in their test files are listed by IPC (available here).
As the relatively recent history of the IPC review process shows in Table 1, a handful of solution providers have supported and continue to support class D FMDs, as well as the other reporting classes A and C (not shown). It may be concluded that there are enough competent software solution providers to offer a choice, yet not be overwhelming for companies just embarking on the journey to generate FMDs. Specific company names can be found on the IPC web site.
Some of the basic requirements for conformance to the standard schema include the following:
- All mandatory data elements (tags) are present, which must be completed when entering data into the tool, if not already present from some prior data entry or load. These elements include data like supplier and customer IDs and part numbers, and homogenous materials in the product broken down by substances and their weights.
- Ability to enter the most useful optional data elements as desired, or as requested by the customer. For example, substance weight is mandatory, but concentration is optional.
- Ability to incorporate a legal statement, either with a standard boilerplate or by entering a custom statement.
- Tags identifying the data as class D (FMD) based on substance reporting at the homogeneous material level; class C substance category reporting at the product level; or class A Query/Reply format (true/false compliance statements).
- Further details including a complete list of Mandatory and Optional data may be found in the IPC-1752A standard.
Requirements Part Two: Lanes of Chemical Data
Since FMD is all about the chemical data, and suppliers in the electronics industry may not have extensive chemical expertise, this set of functionality is critical to generating class D XMLs as correct and error-free as possible. Clearly, to even begin generating FMDs requires having the product’s chemical composition data, and unfortunately there’s no magic to manage this complexity other than a materials and substances database or spreadsheets to manage the list of ingredients to be reported.
Some of the major CAD systems are offering functionality to select raw materials from a database coupled to the CAD system, which is logical since the designer is specifying the materials in the first place. More about how to develop basic “what’s in the product” documentation may be a good topic for another report, since by some accounts this is still the greatest obstacle to begin any FMD.
Next, we briefly highlight important functional requirements for any tool used to generate FMDs from materials and substance data.
Ability to select substances from a list by CAS number prevents errors, since CAS number is a key for many receiving systems and is the Authority tag specified in the IPC standard. A CAS number lookup list should be provided in the tool, which has the advantage of speeding up data entry and selecting matches quickly from valid selections provided by the tool. The tool may also validate the format of the CAS number format itself, which must be 10 digits separated into three groups by hyphens, with the last digit being a check digit. These rules are published by CAS.
This type of validation may be useful to allow newer, valid CAS numbers that aren’t in the lookup table to still be entered. The problem of “wildcard” or declarable/reportable substance lists will be covered in the next section, since it remains a problem in the industry.
A lookup of substance names can also expedite entering substances data, again by ensuring that a valid substance name is used and a valid CAS number goes along with it. The advantage is that a substance name may be more easily recognized and used by a human than a CAS number. This approach to validation has the disadvantage that compounds and even pure elements may have many different synonyms, in which case the direct CAS number lookup would be more useful.
2. Example of material and substance input screen.
The input screen for materials and substances data may look something like Figure 2. This kind of table is the heart of a FMD, where each Material is composed of its constituent Substances, all with reported weights in the product. Optionally, data such as attachment files and concentration ranges may be accommodated. If Exemptions apply at the Material level, they may also be selected.
Direct entry into a reporting tool is likely to be of interest to suppliers making raw materials or simple components, rather than OEMs. But this data becomes the basis of creating FMDs for more complex products. Suppliers of the following kinds of items might be in the best position to take advantage of the kinds of tools being discussed here:
- Solder and solder flux (separately or in paste or wire)
- Bulk material like sheets, or parts made of a homogenous material
- Metal alloys, or parts made from them
- Wire
- Mold compound; molded parts
- Underfill
- Conformal coating
- Plating, painted or dipped coating, or other types of coating
- Adhesives, lubricants or sealants
FMDs for these items can allow the next tier customer to use it for reporting at the next assembly level. Companies on the more complex end of the supply chain may need to use larger enterprise systems to collect this data and run final reports, so the more basic FMD generating tools may be of less interest to them.
Figure 3 shows how the data cascade works. As originally envisioned with the first release of IPC-1752 in 2006, the data cascade is still deserving of more widespread understanding and more thorough implementation today. We realize the complexity of electronic products, since even small personal-use devices may contain hundreds to thousands of components, the key point here is that having good tools at the very beginning stage can be useful as the data builds in complexity up to reports for more complex products.
3. Cascade of FMD data.
Requirements Part Three: The Wildcard Detour
By necessity, companies and standards organizations themselves have used Reportable or Declarable Substances Lists (DSL) for years. One of the early lists was the Joint Industry Guide (JIG), which went through several updates. Amendment 3 to IPC-1752A includes the following statement: The IEC 62474 database of restricted and declarable substances replaced the Joint Industry Guide (JIG) in January 2014.
Meanwhile, most companies have created their own DSLs so that they will receive:
- Data on those substances currently with regulatory restrictions
- Data on industry-specific substances
- Data on other substances the company’s customers expect to know about
- Substances that are not yet under any regulatory restrictions, but could be at some future date.
This latter case is exactly the REACH situation shown at the beginning of this paper. While there are some ways to get advanced information about the next substances to be added to REACH, these are not usually foolproof. Therefore, companies tend to cast a rather wide net to ensure future substances are being included in their suppliers’ declarations.
The IEC62474 database as of this writing was most recently revised on Sept. 3, 2017, and contains 137 Declarable substances and 482 Reference substances. Many companies have nonetheless found it necessary, for the reasons listed above, to develop more comprehensive lists of their own. Without mentioning specific company DLSs here, a general review of some of the many that are used in the electronics industry shows two basic trends:
- Substances listed in common as a core set of substances, including those in common with the IEC62474 list
- Substances that are less frequently found and not part of a common core list
Since homogeneous material reporting is at the substance level, with authority being the CAS number, this presents a data-handling/segregation dilemma.
In a good reporting tool, then, some accommodation must be made for accepting these “wildcard” substances that inherently have no CAS number. We have seen that a commonly, but not universally, accepted limit of 10% per homogenous material can be checked in the tool to ensure this limit is not exceeded. A good tool should also allow for a choice of a customer-specific wildcard substance name, which is not subject to checking from a CAS number list or a CAS format validation routine. Based on our review, if proper warnings are provided, this appears to be the best way at present to handle such data.
Requirements Part Four: Fewer Barriers, More Open Road
So far, we have presented desirable features to help generate FMDs. The real power, though, is only realized through ability to revise, reuse, and build on data once it has been initially entered into a tool.
First of these capabilities is ability to quickly edit the substances in a material. For companies making a family of items with related composition, changing just a few entries, or modifying the percentages of them, can be done in seconds and saved under a different product name and XML file, with version tracking as desired in the file name. This should be allowed during an existing session, or combined with the next feature.
Once a complete XML FMD is generated, productivity is enhanced if that file can be easily imported again at a later date and edited as required. This portability and flexibility of data allows a supplier further down the supply chain to utilize a growing library of common materials and their formulations, and to quickly create new ones without having to repeat some or even most of the data entry. In most cases, the company’s own information, like company ID, contact, and authorizer, will be repeated over many declarations. If contact and authorizer are the same person, they could be copied directly with a choice in the tool.
While we are focusing here on the class D FMD, the ability to generate class A or class D declarations at the same time may be useful. These should be selected or de-selected as desired, or as required by the customer. Class A query/response answers should be straightforward, as should selection of exemptions from a list. Similarly, the substance data in the Class D data section should be quickly erased to enter fresh data while retaining the rest of the information already entered.
Interactive help should provide guidance to first-time and infrequent users. Checks along the way improve speed and accuracy without having to consult a user’s manual, which incidentally should also be easily accessible for those who wish to read the instructions first. Finally, the tool should prevent hang-ups and errors. Support services should be provided, and a process to investigate and resolve bugs, or perceived bugs, should be easy to submit and provide timely responses.
At the end of data entry, there should be a final validation of the XML, included mandatory information has been entered, and the weights of substances add up to each homogenous material being reported. These checks help ensure that the XML will successfully load to the customer’s system.
Conclusions—To the Superhighway
According to some observers, FMD still has too many barriers to really catch on. We disagree. Supply-chain reporting has to begin somewhere, by providing data to middle supply chain companies, and so forth up to chain to the OEM. FMD is the only reporting approach that helps to minimize the ongoing burden of keeping up with the ever-growing lists of regulated substances and expiring exemptions.
We have mentioned that the IEC 62474 database is now invoked as the reportable substance list in IPC-1752A Amendment 3. In addition, development of an IEC 62474 international standard for reporting is in process, which will also specify an XML format. Work is underway to harmonize the schema of both IPC and IEC standards via communication and common participants in both IPC and IEC standards development, but differences should still be expected. Development of the IEC reporting standard nevertheless underscores the interest and need for FMD realization in XML format.
Further enhancements can also be realized in the future. Here’s a few:
- Integration with manufacturing data for mixed, compounded, or formulated materials
- Availability of integrated materials selection with design tools for more complex products
- True Business-to-Business methods to request data as well as provide data all in standard machine-readable formats
- Enhanced error checking, validation of common-sense rules, and agreement between, for example, a class A declaration that says RoHS Compliant = True, yet the class D file for the same item reports a RoHS substance over the threshold percent.
- Support for updated guidance from ECHA on Once an Article, Always an Article, which changes substance percent reporting from any top-level assembly to the lowest-level Article exceeding the 0.1% SVHC threshold. It’s becoming clear that this will require a new data attribute to flag Articles, as distinct from unshaped Materials or Complex Objects. It’s our understanding that this will be considered in the development of a revised IPC-1752B Standard.
In addition to the motivation of staying ahead of the growing number of regulated substances, business processes and procedures that support FMD can also be useful. The expectation that FMDs must be provided can be a requirement for gaining new business or for qualification of an item as a prerequisite to be purchased. A company can also include FMD responsiveness in ongoing supplier evaluation performance ratings that may influence awarding future business. Once an FMD is received in well-formed XML format, loading this data to the customer’s system can be automated for maximum efficiency and reused through the FMD cascade process. We have seen it work and only need more FMDs entering the road.
References
1. M. Myles, “Supply Chain Data Exchange for Material Disclosure.” Journal of Surface Mount Technology, Vol. 19-1, 2006. https://www.smta.org/knowledge/journal_detail.cfm?ARTICLE_ID=123
2. R. Franz, “Beyond “Lead-Free”: An Update on the IPC-1752A Standard for Materials Declaration.” Electronic Design, Jan. 8, 2015. http://www.electronicdesign.com/components/beyond-lead-free-update-ipc-1752a-standard-materials-declaration
3. https://www.assentcompliance.com/fmdcomplete/ © 2017 Assent Compliance Inc.
>> Electronic Design Resources
.. >> Library: Article Series
.. .. >> Topic: System Design
.. .. .. >> Series: RoHS and Critical Materials
About the Author
Roger L. Franz
Environmental Sustainability Advocate and Contributing Author
Roger Franz has served as Principal, Engineering IT at TE Connectivity, Research Engineer at UL Environment, and Group Leader and Design for Environment Manager at Motorola. In addition to product environmental compliance and standards, his passions include new product development, quality, and technology research. His papers have appeared in several industry journals. He has had over a dozen article published in Electronic Design. He holds a BA and MS in chemistry from Grinnell College and Northwestern University, respectively.