University Researchers Pursue Better, Safer Li-Ion Batteries
In a rechargeable lithium-based battery, lithium ions — atoms that have given up an electron, and thus carry a net charge — are pulled out of the battery’s cathode during the charging process, and returned to the cathode as power is drained. But these repeated round-trips can cause the electrode material to shrink and expand, leading to cracks and degrading performance over time.
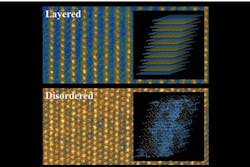
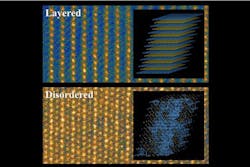
In today’s lithium batteries, those cathodes are usually made of an orderly crystalline material, sometimes in a layered structure. When slight deviations from that perfect order are introduced, the battery’s efficiency generally goes down — so disordered materials have mostly been ignored in the search for improved battery materials.
But it turns out this correlation is far from universal: Certain kinds of disorder can provide a significant boost in cathode performance, the researchers have found through a combination of computer modeling and laboratory experiments. These surprising findings are reported this week in the journal Science, in a paper by MIT graduate student Jinhyuk Lee, professor of materials science and engineering Gerbrand Ceder, and four others.
Ceder describes the materials that can release and then reabsorb the lithium ions as a kind of “reversible sponge.” In today’s batteries, the cathodes are striated materials, made up of lithium layers alternating with oxides of transition metals. Scientists had thought the layering was necessary to provide a pathway for lithium to pass in and out of the cathodes without bumping into the transition metal oxide layer — “a channel with nothing in the way,” as Ceder says.
Moreover, disorder “usually significantly reduces the lithium ion mobility,” Ceder says — and high mobility is essential for an efficient rechargeable battery.
But it turns out that a significant excess of lithium in the material changes things dramatically. In the traditional ordered structure, there is an exact balance between the number of lithium and metal atoms. “But if you get enough of a lithium excess,” Ceder says, “you get new channels, and they can take over from the channels you close off.”
While the disordered material with excess lithium produces irregular pathways, it turns out that these nevertheless can still act as efficient channels for the lithium ions. But such a material offers an extra bonus: While the irregular channels let lithium pass just as easily as it does in a layered material, in the disordered material the lithium ions don’t push the layers out of shape.
The new material — in these experiments, lithium molybdenum chromium oxide — “has a very high dimensional stability,” Ceder says. In most other lithium cathode materials, “as you pull the lithium in and out, it changes dimension, swelling or contracting.” This swelling and contracting “causes all sorts of problems,” including fatigue that can lead to cracking, he says.
While the dimensional changes in layered materials can be as much as 5 to 10 percent, he says, in the new disordered material it is only about 0.1 percent — “virtually zero.”
Ceder stresses that his group’s analysis of this specific compound “shows a new direction that we can take” in searching for even better materials, opening a whole new category of possibilities that had previously been ignored. While lithium molybdenum chromium oxide can hold and release significantly more lithium than existing materials, it produces a lower voltage — meaning its overall performance is about the same as that of existing materials, he says.
Many new materials take decades to move from the laboratory to useful applications, but “we’re hopeful we can do this in one or two years, to discover something better,” Ceder says — most likely by using computational tools such as the Materials Project, which he co-founded.
Jeff Dahn, a professor of physics and atmospheric science at Dalhousie University in Nova Scotia who was not involved in this work, says, “These experimental results are very surprising.” While it remains to be seen “whether this finding can be translated in similar experimental results in more practical materials,” he says, this research “is a nice combination of experiment and theory.”
The research also involved postdocs Alexander Urban, Xin Li, and Geoffroy Hautier at MIT [2008-2009 Total-MIT Energy Fellow] and researcher Dong Su at Brookhaven National Laboratory. It was funded by the Robert Bosch Corporation, Unicore Specialty Optics and Chemicals, Samsung, and the U.S. Department of Energy.
Washington State University Gets in the Act
A group of Washington State University researchers has developed a chewing gum-like battery material that could dramatically improve the safety of lithium ion batteries. Led by Katie Zhong, Westinghouse Distinguished Professor in the School of Mechanical and Materials Engineering, the researchers recently reported on their work in the journal, Advanced Energy Materials. They have also filed a patent.
High performance lithium batteries are popular in everything from computers to airplanes because they are able to store a large amount of energy compared to other batteries. Their biggest potential risk, however, comes from the electrolyte in the battery, which is made of either a liquid or gel in all commercially available rechargeable lithium batteries. Electrolytes are the part of the battery that allow for the movement of ions between the anode and the cathode to create electricity. The liquid acid solutions can leak and even create a fire or chemical burn hazard.
While commercial battery makers have ways to address these safety concerns, such as adding temperature sensors or flame retardant additives, they “can’t solve the safety problem fundamentally,’’ says Zhong.
Zhong’s research group has developed a gum-like lithium battery electrolyte, which works as well as liquid electrolytes at conducting electricity but which doesn’t create a fire hazard.
Researchers have been toying around with solid electrolytes to address safety concerns, but they don’t conduct electricity well and it’s difficult to connect them physically to the anode and cathode. Zhong was looking for a material that would work as well as liquid and could stay attached to the anode and cathode – “like when you get chewing gum on your shoe,’’ she told her students.
Advised by Zhong, graduate student Yu “Will” Wang designed his electrolyte model specifically with gum in mind. It is twice as sticky as real gum and adheres very well to the other battery components.
The material, which is a hybrid of liquid and solid, contains liquid electrolyte material that is hanging on solid particles of wax or a similar material. Current can easily travel through the liquid parts of the electrolyte, but the solid particles act as a protective mechanism. If the material gets too hot, the solid melts and easily stops the electric conduction, preventing any fire hazard. The electrolyte material is also flexible and lightweight, which could be useful in future flexible electronics. You can stretch, smash, and twist it, and it continues to conduct electricity nearly as well as liquid electrolytes. Furthermore, the gummy electrolyte should be easy to assemble into current battery designs, says Zhong.
While the researchers have shown good conductivity with their electrolyte, they hope to begin testing their idea soon in real batteries. Zhong’s group was part of a group of WSU researchers that received support from the Washington Research Foundation last year to equip a battery manufacturing laboratory for building and testing lithium battery materials in commercial sizes. The research groups also are working together to combine their technologies into safer, flexible low-cost batteries.
About the Author

Sam Davis Blog
Editor-In-Chief - Power Electronics
Sam Davis was the editor-in-chief of Power Electronics Technology magazine and website that is now part of Electronic Design. He has 18 years experience in electronic engineering design and management, six years in public relations and 25 years as a trade press editor. He holds a BSEE from Case-Western Reserve University, and did graduate work at the same school and UCLA. Sam was the editor for PCIM, the predecessor to Power Electronics Technology, from 1984 to 2004. His engineering experience includes circuit and system design for Litton Systems, Bunker-Ramo, Rocketdyne, and Clevite Corporation.. Design tasks included analog circuits, display systems, power supplies, underwater ordnance systems, and test systems. He also served as a program manager for a Litton Systems Navy program.
Sam is the author of Computer Data Displays, a book published by Prentice-Hall in the U.S. and Japan in 1969. He is also a recipient of the Jesse Neal Award for trade press editorial excellence, and has one patent for naval ship construction that simplifies electronic system integration.