Lithium-Air Battery Design Poised to Increase Energy Storage, EV Range
What you’ll learn:
- Solid electrolytes are neither volatile nor flammable, unlike the liquid electrolytes used in conventional lithium-ion batteries.
- Discovery of a new lithium-air battery that can store much more energy per volume of battery than today’s lithium-ion designs.
- If commercialized, this design could radically extend the driving range of electric vehicles while significantly reducing battery weight and size.
Given the important role lithium-ion batteries play in the modern world, researchers are continually trying to develop safer and more energy-efficient battery technology.
In a recently published paper, a team led by scientists at the U.S. Department of Energy’s (DOE) Argonne National Laboratory revealed key insights into solid electrolytes that they’re testing for use in all-solid-state batteries.
All-solid-state batteries use solid instead of liquid electrolytes and are neither volatile nor flammable, unlike the liquid electrolytes used in conventional lithium-ion batteries. They’re emerging as a critical technology for the future development of lightweight, energy-dense, longer-lasting, and safer lithium-ion batteries.
This design could radically extend the driving range of electric vehicles while significantly reducing battery weight and size.
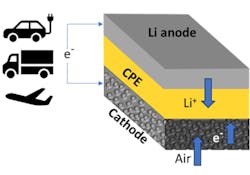
Inside the Lithium-Air Battery
The new battery uses a solid composite electrolyte based on nanoparticles that contain lithium. The electrolyte is embedded in a matrix made of a material called ceramic-polyethylene (CPE) oxide polymer.This solid-state lithium-air battery is the first to achieve a four-electron chemical reaction at room temperature. In a four-electron chemical reaction, four electrons are transferred between two chemical species. This type of reaction, although common in various fields such as electrochemistry, is a feat never before accomplished in a lithium-air battery operating at room temperature. Most lithium reactions involve one or two electrons.
Reactions involving more electrons results in greater energy storage. The four-electron reaction creates lithium oxide (Li₂O) instead of the traditional lithium superoxide (LiO2) or lithium peroxide (Li2O2), both of which limit energy output.
A key element in this chemistry is a powerful catalyst called trimolybdenum phosphide (Mo₃P). This catalyst facilitates the critical four-electron transfer while ensuring the reaction remains stable over long term use.
With the development, the researchers said, their lithium-air design could reach a record energy density of 1,200 Wh/kg. That density is 4X greater than can be found in today’s lithium-ion batteries.
What is LLZO?
The study showed that a solid electrolyte made of lithium lanthanum zirconium garnet (LLZO, or for those in the audience with a degree in chemistry Li7La3Zr2O12) was the best candidate for such a battery. This material stands out because of its strength and durability. It’s also notable for its conductivity, or the ease with which it moves lithium ions between electrodes during charge and discharge.
To make the solid-electrolyte LLZO even better, researchers have been experimenting with adding small amounts of elements like aluminum or gallium to improve how well the LLZO conducts lithium ions. This process is known as doping. Doping means adding small amounts of another element to change and improve the properties of a material.
Doping with aluminum and gallium helps LLZO to retain the most symmetric structure and creates vacant spaces. These spaces allow lithium ions to escape more readily from electrodes and improve conductivity. However, doping can make the LLZO more reactive with lithium metal, shortening the cycle life of the battery. Here, however, researchers showed that the battery can be recharged for at least 1,000 charge-discharge cycles.
In the study, researchers examined what happens when LLZO containing aluminum or gallium dopants contacts metallic lithium. Understanding why the LLZO behaves differently, depending on which dopant has been added, will help scientists design better materials for stable and reliable solid-state batteries.
Impact of Gallium Doping on LLZO
Gallium-doped LLZO is attractive because it has a much higher ionic conductivity than aluminum-doped LLZO. However, the reactivity of these dopants when put in contact with lithium is what led researchers to determine that in order to use gallium, an interfacial layer is needed to protect and preserve its conductivity but prevent its reactivity.
Using computational and experimental techniques, the researchers found that gallium tends to move more easily out of the electrolyte and has a stronger tendency to react with the lithium to form an alloy. This causes the amount of gallium to decrease. The loss of gallium can cause the lithium to change its structure and decrease ionic conductivity. Conversely, aluminum-doped LLZO remains intact.
“It’s important to know how a dopant will react with lithium,” said Peter Zapol, an Argonne physicist and lead researcher on the paper. “It’s another requirement for good electrolytes, not just high conductivity.”
In this case the researchers were able to measure key properties of the doped materials. At the same time, they gained atomic-level insights into what’s happening at the interface between the lithium metal and solid electrolyte.
Using a powerful computer-based method known as density functional theory—a quantum mechanical modeling method to study how atoms and electrons behave in materials—the researchers were able to predict the stability of various dopants and how they would react with other substances.
The lithium-air battery has the highest projected energy storage density of any technology being considered for the next generation of batteries. As such, this technology would dramatically increase how much energy batteries can store. Using a solid-state electrolyte instead of a liquid electrolyte would also dramatically reduce safety concerns due to fire.
Furthermore, this discovery opens up novel ideas for designing a lithium-based battery chemistry that works at room temperature. These future designs could achieve even greater energy storage.