Scandium-Doping Technique Extends Sodium-Ion Battery Life
What you'll learn:
- Sodium-ion batteries are being investigated as a viable cost-effective, more environmentally friendly, and easier-to-source alternative to lithium-ion.
- 60% capacity is retained after 300 cycles.
- Scandium blocks moisture damage and structural breakdown.
Lithium-ion (Li-ion) batteries have been the mainstay for powering EVs, but lithium is a costly and relatively scarce material. Sodium-ion batteries, based on a material abundant in Earth's crust, offer a viable alternative to conventional lithium-ion batteries, which includes their reduced impact on the environment.
Market Research Future, a global market research company, predicts that electric-vehicle application of sodium-ion chemistry is expected to grow from $0.5 billion (USD) in 2024 to $2.5 billion (USD) by 2035.
Choosing the Right Cathode Material
In batteries, the choice of cathode material primarily influences battery capacity and stability. Recently, researchers at the Tokyo University of Science, Japan, published the paper “Unique Impacts of Scandium Doping on Electrode Performance of P’2- and P2-type Na2/3MnO2” in the journal Advanced Materials that investigates layered sodium manganese oxides (Na2/3MnO2) as cathode materials for high-capacity sodium-ion batteries without using any rare-earth metals.
In the past, while these materials exhibited high initial capacity, their capacity faded during charge-discharge cycling, and it’s remained a significant challenge. Here, the researchers examined the key mechanisms through which scandium doping could improve the stability and cycle life of sodium-ion battery cathodes.
Diagnosing the Charge-Discharge Cycling Problem
During charge-discharge cycling of NaMnO2 electrodes, Na+ ions are constantly inserted and extracted from the cathode material. This is accompanied by changes in the oxidation states of manganese between Mn3+ to Mn4+. When Mn3+ ions form, they distort their surrounding lattice to lower electronic energy, a phenomenon known as Jahn-Teller distortion.
Over time, these repeated distortions lead to a buildup of strain at both the atomic and particle level in NaMnO2, eventually resulting in the loss of crystallinity and severe capacity degradation. This is the main cause of capacity loss during cycling of Na2/3MnO2 electrodes.
Long-Term Stability with Scandium-Doped Manganese-Oxide Electrodes
The research team, led by Professor Shinichi Komaba, along with Kodai Moriya and Project Scientist Dr. Shinichi Kumakura, from the Department of Applied Chemistry at Tokyo University of Science, revealed how scandium (Sc) doping can dramatically improve the cycling stability of P'2 polytypes of Na2/3MnO2 electrodes.
"Previously, we discovered that Sc doping in P'2 Na2/3[Mn1-xScx]O2 electrodes can improve the battery performance and long-term stability," explained Prof. Komaba. "However, the exact mechanism for this improvement remains unresolved, and it was unclear whether this effect is generally applicable. In this study, we systematically studied P2 and P'2 polytypes of Na2/3[Mn1-xScx]O2 to understand the role of Sc doping."
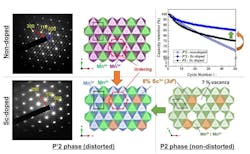
Structural tests revealed that Sc doping in P'2 Na2/3[Mn1-xScx]O2 effectively modulates its structure, resulting in smaller particles and altered crystal growth, while preserving cooperative Jahn-Teller distortion and superstructure. This significantly improves structural stability. In addition, the team found that Sc doping prevents side reactions with liquid electrolytes and enhances moisture stability by forming a cathode-electrolyte interface layer.
As a result, in Na-half-cell tests, the Sc-doped P'2 type Na2/3[Mn1-xScx]O2 electrodes demonstrated a substantial improvement in cycling stability. The sample with 8% Sc doping was found to have optimal performance. The researchers also discovered that unlike non-doped samples, the crystallinity of the doped samples was maintained during cycling.
Interestingly, Sc doping didn’t improve the cycling stability of P2 NaMnO2 electrodes, indicating a specific synergy between Sc doping and cooperative Jahn-Teller distortion. Furthermore, doping with other similar metal cations, like ytterbium and aluminum, didn’t reduce capacity fading, highlighting the unique role of Sc.
"Since Sc is an expensive metal, our study demonstrates its feasibility in the development of batteries. Our findings can potentially lead to development of high-performance and long-life sodium-ion batteries," said Prof. Komaba. "Moreover, beyond sodium-ion batteries, our study illustrates a new strategy to extend the structural stability of layered metal oxides involving the lattice distortion and improve the performance of batteries made using these materials."