Safer, Better Batteries Ahead…Maybe
What you''ll learn:
- Stanford University's redesign of current collectors.
- Texas A&M's look into carbon nanotubes to overcome overheating and failure of lithium batteries.
The technology landscape is cluttered with the promises of presumably “better” batteries that looked promising in the lab but just didn’t work out commercially, assuming there even was an attempt to scale them up to a pilot run prior to actual commercialization. While the reasons for this failure to make the leap are as varied as the battery advances themselves, that doesn’t mean innovators have stopped trying.
Now, two unrelated research teams—one at Stanford University/SLAC and the other at Texas A&M University—have devised what they say will be safer and better batteries. These advances address the double-edged reality of such lithium-based (Li-ion) electrochemical-energy reservoirs—their high energy density and risks associated with this kind of density.
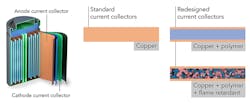
A Stanford team, working with researchers at the Department of Energy’s SLAC National Accelerator Laboratory, has redesigned the thin sheets of copper or aluminum foil in a battery, called current collectors (one of the heaviest battery components), so that they weigh 80% less and immediately quench any fires that flare up. These collectors distribute current flowing in or out of the electrode, and account for between 15% to as much as 50% of the weight of some high-power or ultra-thin batteries.
The team set up experiments for making and testing current collectors based on a lightweight polyimide polymer, which resists fire and stands up to the high temperatures created by fast battery charging. A fire retardant—triphenyl phosphate, or TPP—was also embedded in the polymer; it was subsequently coated on both surfaces with an ultra-thin layer of copper (Fig. 1).
The copper thus fulfills its role of distributing current, but also protects the polymer and its inner fire retardant and is highly effective at suppressing fires and flare-ups (Fig. 2).
The change reduced the weight of the current collector by 80% compared to today’s versions. Postdoctoral researcher Yusheng Ye pointed out that this “translates to an energy density increase of 16% to 26% in various types of batteries, and it conducts current just as well as regular collectors with no degradation.”
Added Yi Cui, SLAC and Stanford professor who led the research, “The current collector has always been considered dead weight, and until now it hasn’t been successfully exploited to increase battery performance. People have also tried adding fire retardant to the battery electrolyte, which is the flammable part, but you can only add so much before it becomes viscous and no longer conducts ions well.”
The work is detailed in their paper in Nature, “Ultralight and fire-extinguishing current collectors for high-energy and high-safety lithium-ion batteries,” along with Supplementary Information and many videos (although the main paper is behind a paywall, there’s a pre-print of it here). The researchers also have applied for a patent. The work was supported by the DoE’s Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office under the eXtreme Fast Charge Cell Evaluation of Lithium-ion Batteries (XCEL) program.
Texas A&M’s CNT Approach
At Texas A&M University, researchers created a technology to prevent lithium batteries from overheating and failing. Their carbon-nanotube (CNT) design for the batteries enables the safe storage of a large quantity of lithium ions, thereby reducing the risk of fire. Furthermore, it will help lithium batteries charge faster than today’s commercially available batteries.
“This new architecture prevents lithium from accumulating outside the anode, which over time can cause unintended contact between the contents of the battery’s two compartments, which is one of the major causes of device explosions,” said Juran Noh, a material sciences graduate student on the team.
The researchers sought to overcome the well-known dendrite problem, where their growth eventually pierces through the material that separates the battery’s two compartments, resulting in a short circuit that can cause the battery to ignite. The growing dendrites also adversely affect the battery’s performance by consuming lithium ions and leaving them unavailable for generating current flow.
The team designed anodes using carbon nanotubes as scaffolds with spaces or pores for lithium ions to enter and deposit. These structures don’t easily bind to lithium ions, though, which discourages dendrite growth but also impedes the ability of the batteries to deliver large currents.
However, additional “fine-tuning” of the CNT anodes with an optimum quantity of the binding molecules prevented the formation of dendrites and enabled a vast quantity of lithium ions to bind and spread along the scaffold’s surface (Fig. 3). This enhanced the battery’s ability to produce large, sustained currents.
As Noh pointed out, “When we had just the right amount of these binding molecules, we could ‘unzip’ the carbon-nanotube scaffolds at just certain places, allowing lithium ions to come through and bind on to the entire surface of the scaffolds rather than accumulate on the outer surface of the anode and form dendrites.”
In their process, they inserted lithium into porous, three-dimensional, CNT structures with their surfaces altered to have lithiophobic, lithiophilic, and hybridized lithiophobic/philic characteristics (“lithio” refers to the material’s ability or tendency to “wet” a surface). Their results showed a high areal capacity of 16 mAh/cm2 along with a current density of 8 mA/cm2, yet without noticeable dendrite formation or volume expansion (another common cell issue of concern).
To analyze the effects of their fabrications, they conducted in-operando studies of voltage/current profiles (in operando means “an analytical methodology where the characterization of materials undergoing reaction is coupled simultaneously with measurement of catalytic activity” and is more advanced than the better-known in situ analysis).
Their work is detailed in a paper with the possibly intimidating title “Understanding of Lithium Insertion into 3D Porous Carbon Scaffolds with Hybridized Lithiophobic and Lithiophilic Surfaces by In-Operando Study,”published in ACS Nano Letters.