At Last, Voltage-Tunable and Adjustable Thermal Conductivity
While we have materials and components with controllable, adjustable electronic and magnetic properties, one related characteristic has thus far eluded in-use adjustment. Thermal conductivity is generally considered “invariant” and set by the specific materials used and the construction of the associated device or component.
But this last barrier may be falling: An MIT team working with Brookhaven National Laboratory has developed what they indicate is the first material with voltage-controllable thermal conductivity, in work supported by the National Science Foundation and the U.S. Department of Energy. Their paper published in Nature Materials with the somewhat obscuring title “Bi-directional tuning of thermal transport in SrCoOx with electrochemically induced phase transitions” (plus Supplementary Information) describes what they used and how they did it, along with the underlying thermal and materials science.
Applications for such adjustable, controlled thermal conductivity could include maintaining a constant component temperature by thermally modulating the heat-flow path. Another could be to build a thermal-storage container that insulates and retains the heat as it’s captured, but can be switched to a highly conductive state when that heat is needed.
The traditional thermal-conductivity premise is that atoms inserted into a material’s lattice act as a source of scattering for thermal carriers, and so can only reduce thermal conductivity. Instead, the project has shown that electrochemical control of oxygen and proton concentration in an oxide provides a new ability to bidirectionally control thermal conductivity.
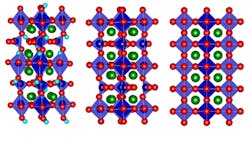
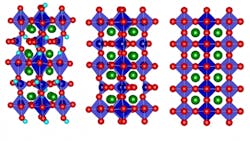
Of course, devising an actual technique for bidirectional control of thermal conductivity isn’t an obvious undertaking. To make it happen, they used a strontium cobalt oxide (SCO), which can be fabricated as a thin film (see figure). By electrochemically oxygenating the brownmillerite (designated SrCoO2.5) to the perovskite (SrCoO3–δ), thermal conductivity increased by a factor of 2.5; in contrast, adding hydrogen to form hydrogenated SrCoO2.5 effectively reduces the thermal conductivity by a factor of four.
In short, adding oxygen to SCO increased its thermal conductivity while adding hydrogen had the reverse effect. The overall tuning of thermal conductivity was across a nearly 10 ± 4-fold range at room temperature, and the variation was greater at lower temperatures.
Thermal conductivity was assessed using time-domain thermoreflectance (TDTR), a technique that can measure its values over many orders of magnitude from a high-end value of ~2000 W/m-K (seen with some diamond and graphite structures) down to as low as 0.03 W/m-K (disordered tungsten diselenide or WSe2 thin films).
How do you add this oxygen or hydrogen as needed? The material is immersed in an ionic liquid (essentially a liquid salt) or put in contact with a solid electrolyte that supplies either negative oxygen ions or positive hydrogen ions (protons) into the material when the voltage is turned on. In the liquid-electrolyte case, hydrolysis of water from the surrounding air is the source of oxygen and hydrogen.
Piecing Together the Physics Puzzle
The underlying physics mechanism was puzzling at first. It was assumed that adding the extra atoms or ions (atoms stripped of some electrons, or with excess electrons), would make conductivity worse due to the greater internal disorder, as thermal energy (heat) tends to be scattered and dissipated by more-irregular atomic structures. However, inserting oxygen ions into the structure of the brownmillerite SCO transforms it into a perovskite structure, which actually has an even more highly ordered structure than the original.
MIT Professor and project leader Bilge Yildiz noted, “It goes from a low-symmetry structure to a high-symmetry one. It also reduces the amount of so-called oxygen vacancy defect sites. These together lead to its higher heat conduction.” The oxygenation/hydrogenation process introduced more order or disorder, respectively, and thus affected thermal conductivity.
Of course, using liquids to adjust the oxygen/hydrogen injection isn’t practical for most systems. Prof. Yildiz pointed out that “what we have shown here is really a demonstration of the concept,” adding that the need for a liquid electrolyte medium for hydrogenation and oxygenation makes this version of the system “not easily applicable to an all-solid-state device.” However, she added that “we know there are solid-state electrolyte materials that, in theory, could replace the liquids” and further investigation is needed.
About the Author

Bill Schweber
Contributing Editor
Bill Schweber is an electronics engineer who has written three textbooks on electronic communications systems, as well as hundreds of technical articles, opinion columns, and product features. In past roles, he worked as a technical website manager for multiple topic-specific sites for EE Times, as well as both the Executive Editor and Analog Editor at EDN.
At Analog Devices Inc., Bill was in marketing communications (public relations). As a result, he has been on both sides of the technical PR function, presenting company products, stories, and messages to the media and also as the recipient of these.
Prior to the MarCom role at Analog, Bill was associate editor of their respected technical journal and worked in their product marketing and applications engineering groups. Before those roles, he was at Instron Corp., doing hands-on analog- and power-circuit design and systems integration for materials-testing machine controls.
Bill has an MSEE (Univ. of Mass) and BSEE (Columbia Univ.), is a Registered Professional Engineer, and holds an Advanced Class amateur radio license. He has also planned, written, and presented online courses on a variety of engineering topics, including MOSFET basics, ADC selection, and driving LEDs.