Biocompatible Protein Nanowires Create Neuromorphic Devices—and Harvest Humidity
What you’ll learn:
- How “green” protein-based nanowires are grown to create neuromorphic devices.
- How these electrically and chemically biocompatible nanowires are used as sensors.
- How these bio-wires also are used to harvest energy from ambient humidity.
“Green” protein-derived nanowires… energy harvesting from ambient humidity… an electronic microsystem that supports basic self-autonomy in a living organism—all of the hot buttons rolled into one project. A research team from the University of Massachusetts (Amherst) has created an electronic microsystem that can intelligently respond to information inputs without any external energy input, much like a self-autonomous living organism. The microsystem, constructed from a novel type of electronics that can process ultra-low electronic signals, incorporates a novel device which can generate electricity “out of thin air” from the ambient environment.
The work was led by Jun Yao, an assistant professor in electrical and computer engineering (ECE) and an adjunct professor in biomedical engineering, working with his longtime collaborator, Derek R. Lovley, a Distinguished Professor in microbiology.
The protein nanowires are harvested from bacteria. Specifically, this project builds on the “Air-Gen” based on microbe Geobacter, discovered by Lovley many years ago, which harvested energy from humidity in the air and was later used to build memristors capable of mimicking human intelligence. (Forgot what “memristors” are? See “Passive Components Get Active” and “Memristor Makes "Moore" Of The Law.”)
Their approach avoids the signal mismatch between the environmental stimuli and driving amplitude in neuromorphic devices that limits the functional versatility and energy sustainability. (Broadly speaking, a neuromorphic device is an electronic analog circuit that mimics the neurobiological architectures present in the nervous system.) The team’s research demonstrated multifunctional, self-sustained neuromorphic interfaces by achieving signal matching at the biological level, using advances that rely on the unique properties of microbially produced protein nanowires, enabling both bio-amplitude (meaning < 100 mV) signal processing and energy harvesting.
Their sensor-driven, integrated neuromorphic systems achieved signal match at the biological level, using three recently recognized properties of protein nanowires produced from this microbe. First, memristors made from protein nanowires can be driven by sub-100-mV signals, thus enabling bio-amplitude neuromorphic circuits and signal processing. Second, devices fabricated with protein nanowires can harvest electric energy from environmental humidity, sustaining signals and energy to computing. Finally, these protein nanowires can function as the sensing component in electronic sensors.
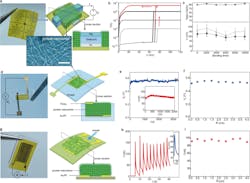
Protein nanowire memristors were fabricated on a flexible substrate (Fig. 1). The device was a vertical structure with an insulating layer (20-nm silicon dioxide) sandwiched between a top (150-nm silver) and a bottom electrode (17-nm platinum). This structure was embedded in a thin layer (~500 nm) of protein nanowire film. The state transitioned to low resistance at 70 (± 3 mV) and spontaneously relaxed to high resistance at close-to-zero bias.
1. (a) (left) Fabricated protein-nanowire memristor arrays on a polyimide (PI) substrate and (right) the schematics of the device structure. (bottom) Transmission electron microscope (TEM) image of protein nanowires. Note that the actual nanowire density is much higher in assembled film. Scale bar, 100 nm. (b) Switching I–V curves from a memristor with the current compliance (ICC) set at different values from 5 µA to 10 nA. (c) Device yield (top), threshold switching voltage (black, bottom), and forming voltage (gray, bottom) in protein nanowire memristors subjected to various bending times. (The error bars represent the standard deviation). (d) (left) A fabricated protein nanowire sensor with a vertical structure as shown in schematics (right). (e) Open-circuit voltage (Vo) and short-circuit current (inset) from the vertical protein-nanowire sensor in the ambient environment (RH ~50%). (f) Output voltage Vo from the vertical protein-nanowire sensor at different bending radius of 4–0.1 cm. (g) (left) A fabricated protein-nanowire sensor with a planar structure as shown in schematics (right). (h) Current and voltage (inset) signals generated in the planar protein-nanowire sensor elicited by breathing. (i) Breathing-induced peak current in a planar protein-nanowire sensor at a different bending radius of 4–0.1 cm.
The team also evaluated active sensors made from protein nanowires, in two device configurations:
- As a vertical structure with a protein nanowire film (~5 µm thick) sandwiched between a top and bottom gold electrode, which was further embedded between two polydimethylsiloxane (PDMS) layers for flexible integration. The protein nanowire film builds up a vertical moisture gradient in the ambient environment for sustained harvesting.
- As a planar structure with a protein nanowire film (~1 µm thick) deposited on a pair of interdigitated electrodes. Due to its in-plane symmetry, this device did not generate a voltage in the ambient environment. However, a quick change of local humidity (from simple breathing-induced non-uniform moisture adsorption) resulted in an instant electric spike.
The energy device was parallel-connected to a resistor (RL) to form a sensor (Fig. 2). The output voltage (Vio) substantially increased from below 30 mV to above 100 mV when the relative humidity (RH) was changed from 50% to 60%. This change in Vio came from the change of the internal resistance (Rs) in the protein nanowires (as Vio = RL/(RL + Rs) × Vo), which was highly sensitive to humidity. As a result, this sensory circuit functioned as an active humidity sensor.
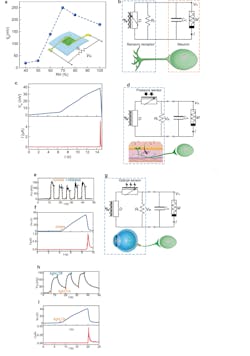
2. (a) Voltage output (Vio) from a vertical protein nanowire sensor connected with a load resistor (RL) measured at different RH. (b) Circuit diagram of feeding Vio from the protein-nanowire sensor (D) into an artificial neuron made from a protein-nanowire memristor (M) and a capacitor (Cm) by emulating an afferent circuit (bottom). (c) Evolution in the membrane potential (Vm) and current (I) in the artificial neuron when placing the sensor on sweating skin. (d) Circuit diagram of feeding Vio from a tactile sensory component (made from a protein nanowire device, D, and a resistive pressure sensor) into an artificial neuron by emulating a tactile afferent circuit (bottom). (e) Touch-induced Vio from the tactile sensory component. (f) Evolution in the membrane potential (Vm) and current (I) in the artificial neuron by pressing the pressure sensor. (g) Circuit diagram of feeding Vio from an optical sensory component (made from a protein nanowire device, D, and an optical sensor) into an artificial neuron by emulating an optical afferent circuit (bottom). (h) Light-induced Vio from the optical sensory component. (i) Evolution in the membrane potential (Vm) and current (I) in the artificial neuron by lighting the optical sensor.
Further, this active humidity sensor was connected to an artificial neuron made from a protein nanowire memristor (M) and a capacitor Cm, with Cm emulating the membrane capacitance in a biological cell. On dry skin (RH < 50%), the sensor had a low output and only charged to a low membrane potential (e.g., Vm < 10 mV), with the memristor remaining Off.
However, when the skin transitioned from dry to sweating after exercise (RH ~90%), the increased Vio charged Cm to an increasing Vm. At Vm ~40 mV, the memristor was turned on and Cm quickly discharged. Thus, the artificial neuron produced a current spike that mimicked the action potential in neuronal firing.
As a final experiment, they developed a protein nanowire sensor that responded only to “instant” RH change. This was used to convert respiration into spiking signals as a frequency-driven neuromorphic interface.
The team concluded that they were able to demonstrate fully self-sustained neuromorphic interfaces based on a “marriage” between bio-amplitude signal processing and environmental energy harvesting, with both enabled by the unique properties in protein nanowires. Their work, which is supported by the Army Research Lab and the National Science Foundation, is detailed in a succinctly titled eight-page paper “Self-sustained green neuromorphic interfaces” published in Nature along with a 20-page Supplementary Information posting.
About the Author

Bill Schweber
Contributing Editor
Bill Schweber is an electronics engineer who has written three textbooks on electronic communications systems, as well as hundreds of technical articles, opinion columns, and product features. In past roles, he worked as a technical website manager for multiple topic-specific sites for EE Times, as well as both the Executive Editor and Analog Editor at EDN.
At Analog Devices Inc., Bill was in marketing communications (public relations). As a result, he has been on both sides of the technical PR function, presenting company products, stories, and messages to the media and also as the recipient of these.
Prior to the MarCom role at Analog, Bill was associate editor of their respected technical journal and worked in their product marketing and applications engineering groups. Before those roles, he was at Instron Corp., doing hands-on analog- and power-circuit design and systems integration for materials-testing machine controls.
Bill has an MSEE (Univ. of Mass) and BSEE (Columbia Univ.), is a Registered Professional Engineer, and holds an Advanced Class amateur radio license. He has also planned, written, and presented online courses on a variety of engineering topics, including MOSFET basics, ADC selection, and driving LEDs.