Download this article in PDF format.
Optical sensing is one of the most prevalent biosensing techniques and, yet, we have only just begun to leverage its full capability. This article uses a photoplethysmography (PPG) sensor to illustrate how an optical sensing system works and discusses possible extensions in new healthcare applications.
What Makes a Biosensor Truly Useful?
To measure a patient’s heart rate, an optical biosensor shines a light into the capillary bed of the patient’s tissue and measures the light that has either traversed or scattered from the tissue. As arterial blood pulsates through capillaries in the tissue, the amount of light it absorbs or scatters changes with each pulse, synchronously to the patient’s heart beat. By observing the variations of light intensity, the optical biosensor can monitor heart rate and other vital signs.
While, at a high level, the principle for optical sensing seems simple, there are a lot of detailed considerations in making a biosensor truly useful.
First, tissues vary among the patient population. A biosensor must make sure that the light reaches the depth it wants to interrogate and that the resulting signal attains sufficient intensity. Approaching this challenge quantitatively, sensor designers create optical models of the tissue by breaking it down to multiple layers, each layer with different refraction, absorption, and scattering properties and thickness (Fig. 1). By simulating a design over different variations of the model, it can be made to better accommodate the variations among a patient population.
1. Shown is an example of modeling human tissue optically in seven layers.
Second, an optical sensor measures changes along the entire optical path. This includes not just the tissue, but also any component packages, air gaps, cover glasses, and the opto-mechanical coupling between the optical sensor and the skin surface. It’s often easier to make sure there is sufficient signal intensity than identifying and removing the “noise” from other factors unrelated to the biosensing signals that affect the optical path. More rigorous designs often employ a series of phantoms, simulacrums of the target tissues that could be subjected to real conditions, and investigate corner cases.
Sensing System Noise
Whether a PPG waveform is used to make simple heart-rate measurements or is fed into an analytics program to derive actionable information about a patient’s wellness, it’s important to understand the source of noise and its statistical impact on the PPG signal.
In a PPG, the system generates a train of light pulses that are received at photodetector(s) after the light passes through the analyte. The intensity of the light pulses and their wavelengths affect the photocurrent received at the photodetector. Fig. 2 (top) shows the photocurrents induced by four LEDs with different wavelengths. Each set of photocurrents can be described as a small alternating signal, which is often called a PPG signal (Fig. 2, bottom), riding on a much larger offset or dc signal.
2. Received signals at an optical sensor come from four LEDs of different wavelength driven with the currents of the same magnitude (top). In the bottom image, the signals from the top portion have their offsets removed.
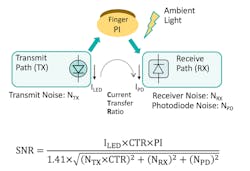
Consequently, one could model a PPG system as a current transform function (Fig. 3) with the LED current as its input and the photodetector current as output. The signal-to-noise of such a system would then be a function of a perfusion index (PI), where PI is the ratio of the magnitude of the PPG signal and the underlying dc signal.
Maximizing PI is the key consideration for industrial designers when they’re creating the physical housing of a PPG sensor. Because light interacts with analytes in the tissue, PI is better when the design includes longer optical path length to allow greater interaction.
3. Here’s a PPG signal path and signal-to-noise ratio.
The area on a patient’s body where the sensor attaches is also a concern. That’s because tissues rich in capillaries would allow greater interaction between the light and the analyte, thus improving PI. PI is worse when air gap and/or cover glass that poorly matches the refractive index of tissue would also attenuate more LED light before it can interact with the analyte.
While PI is important when designing the form factor of a PPG sensor, it’s only one potential source of system noise. Any uncompensated system behavior, such as nonlinearity in LED drive current, will couple unintended variation into the PPG signal, which is a function of the current setting instead of the monitored biology. Furthermore, as human pulse rate is nominally only 100 beats per minute, low-frequency power-supply noise that affects the LEDs or the datpath circuits could appear in band with the PPG signal. Higher-frequency noises could be aliased into sampled results if anti-aliasing measures are inadequate.
Ambient temperature can affect the emissivity of LEDs and result in lower emission intensity at higher temperatures. A change in LED light intensity would affect both the PPG signal and its underlying offset. As such, biosensing results that primarily use PI values, such as pulse oximetry, aren’t significantly affected by ambient temperature so long as the changes in emissivity of the LEDs involved are substantially similar.
Robustness over changing ambient temperature and its non-intrusive nature make PPG sensors a favorite choice among body-worn biosensing systems. Integrated analog front-end ICs for PPG sensors, including Maxim’s MAX8614x, can help reduce the PPG form factor and minimize the effects of potential system noises. They also mitigate against the false signals that can result from fast-changing ambient light conditions; for example, scenarios when a wearer is cycling though woods and, thus, the wearable device is alternately under sunlight or in the shade.
Opportunities for Machine Learning
In a recent paper, a team of researchers at Stanford University studied long-term sensor data (PPG and skin temperature) from wearable devices on 43 adult wearers. The paper suggests that machine-learning algorithms can use sensor information to detect an inflammatory response earlier than the wearer would otherwise notice symptoms and could even distinguish physiological differences between insulin-sensitive and resistive individuals.
In a way, sensing the human pulse to diagnose a patient’s wellness is a very old practice. Doctors in traditional Chinese medicine have been taking their patients’ pulses to make their diagnoses for a very long time. By 1600, Chinese medical literature had listed 28 classifications of pulses. In addition to pulse rates, the literature took note of the differences of pulses taken from the left versus the right wrist, the pulses taken with light versus heavy pressure on the artery, the vigor, and the position of a peak within a pulse period.
More recently, researchers have tried to reconcile traditional practices and pulse classifications with measurement results from modern equipment, but a large gap remains in mapping traditional classifications to different features on a PPG or ECG waveform. Of course, PPG and ECG are just a one-dimensional projection of the multi-dimensional phenomena of a human pulse. It’s reasonable to think that we still need more sensing modalities to fully comprehend the validity of these empirical results accumulated from observations over many hundreds of years.
Modern medicine is also making common use of the PPG. Heart rate and blood-oxygen saturation are among the standard vital signs taken on each patient in clinical settings. A cursory survey of academic papers shows many studies using PPG to estimate arterial mean pressure and hematocrit, while heart-rate variability has been used to measure a patient’s stress levels. PPG and optical methods are not only used to interrogate capillary beds in tissues, but also arteries. Other optical methods have been employed, some using coherent light sources and others involving implants, to monitor post-surgery patients.
The Best Sensors are Invisible
In the final analysis, sensors are useful to us only when they provide actionable information. Consider the weather forecast. We immediately understand our wardrobe options when the report predicts 78°F (or 23°C for non-America readers) and 80% relative humidity. Likewise, we use terms like heat index and wind-chill factors to convey complex interactions between the environment and the consumer in ways that consumers have been educated to understand.
To most consumers, it’s completely invisible that the weather forecast depends on a series of sensors, including temperature, pressure, wind velocity, and relative humidity, as well as very sophisticated models and algorithms. What’s important is that we have developed a set of vocabulary and shorthand to convey a set of complex information that consumers can understand and act upon.
The truly useful biosensor must be a part of a larger information system that provides actionable information for consumers to improve their wellness. But, perhaps, a better understanding of the information we can get using non-intrusive, miniaturized, and inexpensive sensors is a good place to start.
Ian Chen is Executive Director, Advanced Sensors, Industrial & Healthcare Business Unit at Maxim Integrated.
References:
Easson Craig. “Designing accurate, wearable optical heart rate monitors.” MaximIntegrated.com (retrieved July 3, 2018).
Reynolds, K. J. and J. P de Kock, L. Tarassenko and J.T. B. Moyle. “Temperature Dependence of LED and its theoretical effect on pulse oximetry.” British Journal of Anaesthesia 67 (1991): 638–643
Li, Xiao and J. Dunn, D. Salins, G. Zhou, W. Zhou, S. M .Schusler-Fiorenza Rose, D. Perelman, E. Colbert, R. Runge, S. Rego, R. Sonecha, S. Datta, T. McLaughlin, M. P. Snyder. “Digital Health: Tracking Physiomes and Activity Using Wearable Biosensors Reveals Useful Health-Related Information.” PLOS Biology 15 (2017): 1–30.
Tang, Anson Chui Yan. “Review of Chinese traditional pulse diagnosis quantification.” Complementary Therapies for the Contemporary Healthcare (2012): 61–79.