Book Review: “High Temperature Solid Oxide Fuel Cells for the 21st Century” (2nd Edition)
High-temperature solid oxide fuel cells for the 21st Century: Fundamentals, design and applications is edited by Kevin Kendall and Michaela Kendall, Metallurgy and Materials, University of Birmingham (UK) The book includes 16 contributors from the UK, United States, Japan, Poland, China, and Germany.
The book says that solid oxide fuel cells (SOFCs) are the most efficient devices yet invented for conversion of chemical fuels directly into electrical power. It points out that improvements in theory and experiment are still being made 120 years after the original work on fuel cells. During the last decade, significant progress has been made to improve the materials, to construct better systems, to focus on simpler fuels and to seek premium applications.
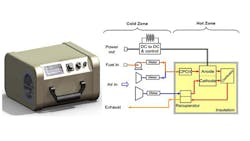
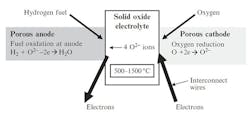
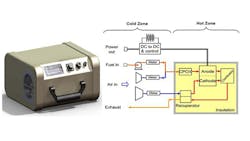
SOFCs contain a solid oxide electrolyte made from ceramic such as yttria stabilized zirconia (YSZ) which behaves as a conductor of oxygen ions at temperatures from 500°C to 1500°C (Fig. 1). This ceramic material allows oxygen atoms to be reduced on its porous cathode surface by electrons brought in through the metal interconnect, thus being converted into oxygen ions, which are then transported through the ceramic oxide electrolyte to a fuel-rich porous anode zone where the oxygen can react, say with hydrogen, giving up electrons to an external circuit. Only five components are needed to put such a cell together: electrolyte, anode, cathode, and two interconnect wires, although other layers are often added.
The polymer electrolyte membrane fuel cell (PEMFC) differs from the SOFC. It uses a water-based, acidic polymer membrane as its electrolyte, with platinum-based electrodes. A major operating difference is that PEMFC cells operate at relatively low temperatures (below 100 °C) and can tailor electrical output to meet dynamic power requirements. Operation at relatively low temperatures and use of precious metal-based electrodes, requires these cells to operate on pure hydrogen. PEMFC cells are mainly intended for light duty vehicles and materials handling vehicles, and to a lesser extent for stationary and other applications.
Although hydrogen would be ideal for an SOFC, it is not the preferred fuel. Hydrogen would more likely be used in a PEMFC, which gives extra power at room temperature. Hydrocarbon gases such as methane, or propane derived from pipelines or liquid stores, or biofuels like methanol and formic acid are more attractive for SOFCs for several reasons:
- They can potentially be reacted directly on the anode; they would be impossible for use with PEMFCs at room temperature with existing catalysts.
- Hydrocarbons react well with steam or air to provide hydrogen and carbon monoxide which then combine with oxygen ions at the anode/electrolyte interface.
- The thermodynamic efficiency of such internal reforming processes can be impressive.
- Hydrocarbon fuels give good energy storage density compared to hydrogen, especially when liquid, for example, propane/butane pressurized at room temperature or methane below its critical point of -83°C.
Advantages of the SOFC compared to PEMFCs are:
- Direct use of liquid fuels which have much superior energy storage capacity (e.g. a factor 6) when compared with compressed hydrogen gas.
- Availability of high-quality heat that can be used to advantage in some practical applications.
Examples of applications demanding this heat are domestic homes which need both power and heat, auxiliary power units (APUs) on vehicles and stationary power generation. In each of these cases, the electrons are extracted from the fuel in the fuel cell at a temperature around 600–1000°C, as shown in Fig. 1. A domestic system would then use the heat to produce hot water as currently achieved with simple heat exchangers. For example, the heat could be used to warm a vehicle. A stationary power generation system may use the waste heat to drive a heat engine such as a Stirling or gas turbine motor.
It is essential to realize that the heat is inevitably generated in the SOFC by reaction exotherm, ohmic losses, electrode overpotentials, etc. These losses are present in any fuel-cell arrangement and cannot be eliminated completely. Indeed, the heat is necessary to maintain the operating temperature of the cells, replacing the heat lost by thermal conduction and radiation to the surrounding environment. The benefit of the SOFC over competing fuel cells is the high temperature of the exhaust heat, making its utilization simple and economic. Thus both electricity and heat are desirable products from the SOFC. The ratio of power to heat can realistically be adjusted from 20% to about 70% depending on the system design, but for basic designs, range from 40% to 60%. These figures compare with about 20–27% for an automobile combustion engine, about 50–60% for the best combined cycle gas/steam turbine power plant and around 40–60% for a diesel combustion generator combined with a steam cycle.
From these basic principles, it is possible to recognize the issues that remain to be solved if SOFCs are to become successful in this century.
Problems to be resolved
The SOFC process is almost magical in its elegance and simplicity, and it is surprising that this process has not yet been commercialized to supplant the inefficient and polluting combustion heat engines which currently dominate.
There are several technical reasons why SOFCs have not yet competed successfully with combustion devices, but cost and durability issues also need attention.
- Combustion has been accepted for several thousand years, and such established technologies are difficult to supplant.
- Combustion does not require any additional materials or interfaces; it can occur freely once ignition of the fuel has occurred. Containment and expansion of the hot gas product is the only requirement for power production. Contrast this situation with the SOFC that needs five materials and four interfaces, each of which can block the power output when contaminated with a single molecular layer of silica.
- Combustion can occur with almost any oxidizable substance so that cheap available complex fuels, for example, petroleum or biomass, may be used. Purity is crucial to fuel cells but has not yet been a barrier to combustion, except where emissions legislation has been imposed.
Progress has been made in several areas, which are covered. Materials invention covered in Chapters 1 through 7 explain how new electrolyte compositions, such as scandia-doped zirconia; new cathodes such as LSCF and new interconnects like ferritic stainless steel alloys have shown better performance. These chapter subjects are:
1. Introduction to SOFCs
2. History
3. Thermodynamics
4. Electrolytes
5. Anodes
6. Cathodes
7. Interconnects
Stack inventions like m-SOFCs described in Chapters 8-10 have allowed smaller and more rapid starting devices to be proven. Subjects include:
8. Cell and stack design, fabrication and performance
9. System designs and applications
10. Portable early market SOFCs
Application projects covered in Chapters 11-13 have taken new directions such that shipments and installations of SOFCs have risen exponentially, and the best example being the many thousands of SOFC CHP generators now installed in Japan.
SOFC systems in the 1–500 W power range have been considered and developed for mobile and portable applications. Examples of mobile/portable applications are power for consumer electronic devices, battery charging, remote power and low-level auxiliary power. Portable SOFC systems are commonly operated on hydrocarbon fuels and particularly suitable for tactical military applications (e.g., soldier power and unmanned vehicle power) due to its potentiality for operation on logistics fuels such as JP-8.
Factors critical in the design of SOFC power systems for portable applications are:
- Stack design
- Stack power density
- System thermal management
- Reforming process selection
- Reformer design
11. Sources of cell and electrode polarization losses in SOFCs
Many barriers remain to our deeper understanding of SOFC electrode reactions. A growing body of research suggests that the key gas/solid and solid/solid interfaces involved in the reaction have compositions and structures that deviate substantially from the bulk, and which are highly sensitive to the exact composition and process history of the electrode. In order to understand electrode reactions at a deeper level, we need a new set of experimental and modelling techniques that provide much more localized information (0.1-100 nm).
12. Testing of electrodes, cells and short stacks
This chapter considered the main types of electrochemical tests that have been applied to SOFCs and outlined the main issues that require detailed attention for obtaining meaningful test results. One important aspect in electrode testing is to assure a correct geometry in three-electrode setups, very difficult in practice in case of electrode supported cells with thin electrolytes. Unfortunately, even testing the individual electrodes in sound geometry set-ups is not a perfect procedure either, because the sum of the contributions from individual cell components to the cell resistance does not add up to the actual measured total cell resistance. This is probably due to differences in the fabrication of the special cells for electrode characterization and the practical cells. It is recommended that cell test results be reported in a way that makes it easy to derive ASR from the i–V curves.
13. Cell, stack and system modeling
14. Fuels and fuel processing in SOFC applications
There is much interest at present in the development of anodes that are resistant to carbon deposition at lower oxidant/carbon ratios in the fuel feed. The development of such anodes is particularly important for SOFCs using co-fed oxygen as the oxidant or small quantities of steam added to the fuel. Furthermore, the possibility of developing SOFCs with such anodes that can run directly on practical hydrocarbon fuels, without any co-fed oxidant, something that has long been regarded as an ultimate goal for SOFCs, is now receiving renewed and vigorous effort, with some encouraging results.
An important consideration for fuel cell systems is the thermal cyclability of the fuel processing catalyst. A fuel cell system is expected to undergo a number of thermal cycles during its operating life. The number of thermal cycles over the lifetime of the system is strongly applications-dependent, with up to 2000 cycles for APUs and portable systems and 20–50 cycles for stationary systems (the pre-reformer is expected to last 5 years). For the Ni-based SR catalyst, the challenge is to avoid deep oxidation of the catalyst during the regime of shutdown where fuel is turned off. A mild surface oxidation is beneficial to protect the catalyst by forming a passive surface layer that can be easily converted to the metal during heat-up to reactivate the catalyst.
The book can be purchased on http://store.elsevier.com/ by searching for the author or title.
Release Date: November 2015
Print ISBN: 978-0-12-410453-2
eBook ISBN: 978-0-12-410483-9
Imprint: Academic Press
Pages: 520
About the Author

Sam Davis
Sam Davis was the editor-in-chief of Power Electronics Technology magazine and website that is now part of Electronic Design. He has 18 years experience in electronic engineering design and management, six years in public relations and 25 years as a trade press editor. He holds a BSEE from Case-Western Reserve University, and did graduate work at the same school and UCLA. Sam was the editor for PCIM, the predecessor to Power Electronics Technology, from 1984 to 2004. His engineering experience includes circuit and system design for Litton Systems, Bunker-Ramo, Rocketdyne, and Clevite Corporation.. Design tasks included analog circuits, display systems, power supplies, underwater ordnance systems, and test systems. He also served as a program manager for a Litton Systems Navy program.
Sam is the author of Computer Data Displays, a book published by Prentice-Hall in the U.S. and Japan in 1969. He is also a recipient of the Jesse Neal Award for trade press editorial excellence, and has one patent for naval ship construction that simplifies electronic system integration.
You can also check out his Power Electronics blog.