Commercial fuel cells are available, but for wider use they need more R&D to improve their performance, reliability, and cost.
To meet this need, R&D at several U.S. universities and national laboratories is focused on making fuel cells a more widely used energy source. Some of this R&D is in the basic research phase, some is more advanced. This R&D is complex because it combines chemical and electrical disciplines, so it needs scientists with an understanding of both. And, sometimes it is hard to find people with this inter-disciplinary background.
One of the current R&D facilities is the University of Delaware’s Center for Fuel Cell Research (CFCR), a resource for innovative energy technologies under the umbrella of the University of Delaware Energy Institute (UDEI). UDEI is the driving force for interdisciplinary research and education on alternative energy at the university and encompasses a number of prominent institutes and centers on campus, including the CFCR.
Ajay Prasad, director of the CFCR, says, “Hydrogen-powered polymer electrolyte membrane fuel cells have already demonstrated the potential to replace internal combustion engines in vehicles and to provide power in stationary and portable applications.” He notes that a major challenge to commercialization of this technology is the durability of the membrane, which is typically made from a polymer called Nafion. During fuel-cell operation, the membrane undergoes chemical and mechanical degradation, leading to cracks and pinholes that shorten its life.
To address this issue, Prasad and two colleagues from the UD Department of Mechanical Engineering, Liang Wang and Suresh Advani, have developed a self-healing membrane incorporating microcapsules prefilled with a Nafion solution. A patent application has also been filed.
“The microcapsules are designed to rupture when they encounter defects in the membrane and then release the prefilled Nafion solution to heal the defects in place,” Liang Wang explains. Fig. 1 shows Wang working on the new membrane material.
Durability testing of the developed membrane has confirmed that the self-healing functionality could greatly extend its useful life.
This research was supported by the University of Delaware’s Fuel Cell Bus Program, which is funded by the Federal Transit Administration.
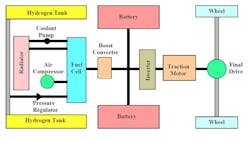
The fuel-cell hybrid electric transit bus is a completely electric vehicle, with no combustion and no generated harmful emissions (Fig. 2). It is a battery-heavy hybrid in which batteries are used as the primary source of electric power for the drive motor, while the fuel cell stack is used to slowly recharge the batteries. Therefore, the fuel cell acts a range extender.
Figure 3 shows the bus system diagram. When the bus is turned on, electricity flows from the batteries to the traction motor, powering the final drive which turns the rear wheels. During operation, if the battery state-of-charge drops below 60%, the fuel cell turns on and provides electricity to the system, to help power the bus and/or recharge the batteries. The bus is equipped with regenerative braking so that when the brakes are applied, the kinetic energy of the bus is converted back to electricity by the traction motor and stored in the batteries.
University of South Carolina
The University of South Carolina includes the Hydrogen and Fuel-Cell Center located in the Department of Chemical Engineering. This research center focuses on the integrated study of fuel cells used for electrochemical power sources. The research covers a number of specific areas related to fundamental knowledge, technical applications, and integrated systems, including:
- Multi-phase CFD Fuel Cell Models with Lattice Boltzmann Method Implementation for High Current Density Operation in PEMFCs (Proton Exchange Membrane Fuel Cells)
- Effect of Contaminants on the Performance of a PEM Fuel Cells
- Transport Studies and Modeling in PEM Fuel Cells
- Characterization of Gas Diffusion Layer and Their Effects on PEMFC Performance
University of California, Irvine
At UC Irvine, The National Fuel Cell Research Center (NFCRC) investigated the feasibility of using a solid oxide fuel cell (SOFC) and a gas turbine (GT), known as an SOFC-GT technology in locomotives.
With state-of-the-art specifications for system sizing, the NFCRC has determined that it is technically feasible to design, build, and install a sufficiently powerful hybrid SOFC-GT system in a long-haul locomotive engine compartment of the future.
NFCRC researchers also developed dynamic simulation and control capabilities for the SOFC-GT locomotive operating on diesel fuel, natural gas, and hydrogen, and established that a hybrid SOFC-GT locomotive could be controlled and operated in the typical fashion to power a locomotive over a high elevation and steep grade.
The NFCRC received funding from the Federal Railroad Administration in collaboration with the U.S. Environmental Protection Agency, the U.S. Department of Energy, the California Air Resources Board, and the South Coast Air Quality Management District to complete a prototype design analyses, identify technology partners to construct a prototype for testing and plan, and prepare a prototype test of the hybrid SOFC locomotive concept.
Georgia Tech
Researchers in the Georgia Tech Research Institute’s (GTRI) Center for Innovative Fuel Cell and Battery Technologies believe that understanding how and why fuel cells fail is the key to both reducing cost and improving durability.
Center director Tom Fuller has been trying to solve what he deems the top three durability problems since he joined GTRI from United Technologies in 2004. “My philosophy is that if we can really understand the fundamentals of these failure mechanisms, then we can use that information to guide the development of new materials or we can develop system approaches to mitigate these failures,” said Fuller, who is also a professor in Georgia Tech’s School of Chemical & Biomolecular Engineering (ChBE).
One of the problems Fuller is addressing includes the chemical attack of the membrane. In a typical fuel cell, hydrogen is delivered to the anode side of the cell that contains a catalyst, such as platinum. The platinum splits the hydrogen molecules (H2) into hydrogen ions and electrons. On the cathode side of the fuel cell, an oxidant such as a stream of oxygen or air is delivered.
With a proton exchange membrane in the middle, only hydrogen ions can travel through the membrane to the cathode. Electrons travel on a different path through the electrical circuit to the cathode, creating an electrical current. At the cathode, the hydrogen ions combine with oxygen and the electrons that took the longer path to form water, which flows out of the cell.
Fuller’s research shows that the membrane, commonly made of a synthetic polymer, is prone to attack by free radicals that create holes in the barrier. The free radicals are formed by the decomposition of hydrogen peroxide (H2O2), a strong oxidizing chemical that can form near the membrane. Perhaps the work done at the University of Delaware can solve this problem.
Another challenge with low-temperature fuel cells is that a blockage can occur on the anode side of the fuel cell, possibly from a water drop formed in the fuel channel. The blockage causes carbon (used to support the platinum) to corrode, turn into carbon dioxide, and leave the fuel cell as a gas. Frequently starting and stopping the fuel cell also causes this mode of failure. This can be catastrophic for the fuel cell because without carbon, the platinum catalyst layer collapses and disappears. “If this happens, the fuel cell can be destroyed in days rather than years,” noted Fuller.
This problem is more common in non-stationary fuel-cell applications, such as cars that require the fuel cell to start and stop when the vehicle is turned on and off.
Another problem with fuel cells cycling on and off is that platinum has a small but finite solubility in the acidic membrane given the high electrical potential and oxidizing environment at the cathode.
“Platinum is one of the most expensive parts of the fuel cells, so researchers study how to decrease the amount necessary to run a fuel cell,” explained Fuller. “But if there is less platinum in the fuel cell to begin with, you can’t afford to lose any by it dissolving.”
NREL
The National Renewable Energy Laboratory (NREL) has acquired four Fuel Cell Hybrid Vehicle-Advanced (FCHV-adv) sport utility vehicles on loan from Toyota (Fig. 3). Over the next two years the lab will use the FCHVs, also known as fuel-cell electric vehicles or FCEVs, to research overall vehicle and fuel-cell system performance, renewable hydrogen production capabilities, fueling infrastructure, and hydrogen energy system integration in real-world conditions. Previous demonstration projects have already shown that FCEVs have double the efficiency of internal combustion engine vehicles and that some have up to a 430-mile driving range.
The FCHVs give NREL a hands-on opportunity to study the integration of renewable hydrogen production with vehicle fueling and energy storage, and to assess the technology for deployment on a commercial scale. The vehicles are fueled with hydrogen produced by renewable electrolysis at NREL’s Distributed Energy Resources Test Facility—wind turbines and solar panels power electrolyzers that produce hydrogen from water, and the hydrogen is dispensed into the vehicles at NREL’s existing hydrogen fueling station. One research area is to determine whether hydrogen production can keep up with demand or if supplemental hydrogen is needed from another source. Another important activity is determining how the vehicles and onboard storage systems interact with the hydrogen fueling infrastructure.
ORNL
Oak Ridge National Laboratory’s research addresses the barriers facing the development and deployment of hydrogen and fuel cells. Through collaborative research and development, ORNL is creating materials and technologies to establish a hydrogen infrastructure and for hydrogen storage onboard vehicles. As part of these efforts, researchers are developing steel-concrete composite underground storage tanks and low-cost carbon fiber.
ORNL is the Department of Energy's (DOE) leading resource for characterization of fuel cell materials through electron microscopy and X-ray photoelectron spectroscopy. Researchers also conduct studies of infrastructure deployment scenarios and fuel-cell vehicle market analysis to provide data for industry leaders and policy makers to use in strategic decision-making.
About the Author

Sam Davis
Sam Davis was the editor-in-chief of Power Electronics Technology magazine and website that is now part of Electronic Design. He has 18 years experience in electronic engineering design and management, six years in public relations and 25 years as a trade press editor. He holds a BSEE from Case-Western Reserve University, and did graduate work at the same school and UCLA. Sam was the editor for PCIM, the predecessor to Power Electronics Technology, from 1984 to 2004. His engineering experience includes circuit and system design for Litton Systems, Bunker-Ramo, Rocketdyne, and Clevite Corporation.. Design tasks included analog circuits, display systems, power supplies, underwater ordnance systems, and test systems. He also served as a program manager for a Litton Systems Navy program.
Sam is the author of Computer Data Displays, a book published by Prentice-Hall in the U.S. and Japan in 1969. He is also a recipient of the Jesse Neal Award for trade press editorial excellence, and has one patent for naval ship construction that simplifies electronic system integration.
You can also check out his Power Electronics blog.