Solid Electrolyte May Improve Rechargeable Li-based Battery Performance
Lithium-based rechargeable batteries are the power-storage medium of choice in many applications due to their very high power/weight and power/volume ratios. Most of today’s lithium-ion batteries use a liquid electrolyte sandwiched between two electrodes. However, these electrolytes can be flammable, and they’ve been responsible for some fires and other major mishaps.
Further, they’re also prone to the formation of “dendrites,” which are thin, fingerlike projections of metal that build up from one electrode and can create a short circuit if they reach all the way across to the other electrode. Not only can these damage the battery, of course, but they also induce high current flows and self-heating. There have been attempts to use a solid electrolyte instead of liquid, which could avoid safety issues while boosting capacity, but these have had their own short-circuit problems and exhibit erratic performance.
Now, a multi-institutional team has advanced a different interpretation of a common failure mode. Their report in Advanced Energy Materials provides evidence that it’s the smoothness of the surface that matters most for long-term performance, a supposition that may lead to greatly improved solid-electrolyte batteries.
The researchers found that any microscopic nicks and scratches on the surface of solid electrolyte can provide a crevice or “toehold” into which the undesired metallic dendrite deposits can migrate, reside, and then further grow. This contrasts with conventional thinking, which was based on the premise that the material’s shear modulus (its firmness or “squishiness”) determined whether dendrites could penetrate into the electrolyte.
Smoothing Away the Dendrites
Yet-Ming Chiang, Kyocera Professor of Ceramics at MIT and one of the project leaders, noted that simply focusing on achieving smoother surfaces could eliminate or greatly reduce dendrite formation in batteries with a solid electrolyte. This may make it possible to use a solid-lithium metal electrode, which could potentially double a lithium-ion battery’s energy capacity.
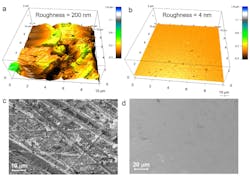
Research suggests that smoother surfaces for the solid electrolyte of lithium batteries could greatly reduce or even eliminate battery-damaging dendrite formation. The figure shows shows a false-color detail of typical solid-electrolyte surface roughness (a); the same detail for the smoother electrolyte used for the tests (b); potential dendrite-formation sites (c) for the surface of image (a), and sites (d) for surface of image (b). (Source: MIT)
“The formation of dendrites, leading to eventual short-circuit failures, has been the main reason that lithium-metal rechargeable batteries have not been possible,” explains Chiang. (Note that lithium-metal electrodes are used in non-rechargeable batteries, but that’s because dendrites only form during the charging process.)
The problem of dendrite formation in lithium rechargeable batteries was first recognized in the early 1970s, says Chiang, “and 45 years later that problem has still not been solved.” Research in solid electrolytes has focused on the assumption that the material needed to be stiff, not elastic. Chiang goes on to say that the idea made sense, as a stiffer material should be more resistant to something trying to press into its surface.
The team tested samples of four solid electrolyte materials and showed the dendrites actually form in stiff solid materials, via a completely different process than those that form in liquid electrolytes. On the solid surfaces, lithium from one of the electrodes begins to be deposited, through an electrochemical reaction, onto any tiny defect that exists on the electrolyte’s surface, including tiny pits, cracks, and scratches (see the figure). Once the initial deposit forms on a defect, it continues to build. (Surprisingly, the buildup extends from the dendrite’s tip, not from its base, as it forces its way into the solid, acting like a wedge as it opens an ever-wider crack.)
These materials are “very sensitive to the number and size of surface defects, not to the bulk properties” of the material, says Chiang. “It’s the crack propagation that leads to failure. We should be focusing more on the quality of the surfaces, on how smooth and defect-free we can make these solid electrolyte films.”
The research team also included Lukas Porz, Tushar Swamy, Daniel Rettenwander, and Harry Thomas at MIT; Stefan Berendts at the Technical University of Berlin; Reinhard Uecker at the Leibnitz Institute for Crystal Growth in Berlin; Brian Sheldon at Brown University; and Till Fromling at the Technical University of Darmstadt, Germany.
About the Author

Bill Schweber
Contributing Editor
Bill Schweber is an electronics engineer who has written three textbooks on electronic communications systems, as well as hundreds of technical articles, opinion columns, and product features. In past roles, he worked as a technical website manager for multiple topic-specific sites for EE Times, as well as both the Executive Editor and Analog Editor at EDN.
At Analog Devices Inc., Bill was in marketing communications (public relations). As a result, he has been on both sides of the technical PR function, presenting company products, stories, and messages to the media and also as the recipient of these.
Prior to the MarCom role at Analog, Bill was associate editor of their respected technical journal and worked in their product marketing and applications engineering groups. Before those roles, he was at Instron Corp., doing hands-on analog- and power-circuit design and systems integration for materials-testing machine controls.
Bill has an MSEE (Univ. of Mass) and BSEE (Columbia Univ.), is a Registered Professional Engineer, and holds an Advanced Class amateur radio license. He has also planned, written, and presented online courses on a variety of engineering topics, including MOSFET basics, ADC selection, and driving LEDs.