Reducing the manufacturing cost of Li-ion batteries and extending their operation time remain very challenging tasks. To attack those problems, engineers are taking one of two approaches.
The first approach involves new engineering designs. Battery designers are developing battery formats that use more energy-producing active materials (anode and cathode), while reducing required, yet space-eating, inactive materials.
For instance, a flat battery format now prevails over round formats, since it allows for more efficient use of real estate. Also being examined is the use of thinner and lighter current collectors, separators, and packaging materials to reduce weight and increase the space available for active materials. Finally, lots of study has gone into new thermal-management designs to improve battery temperature control and eliminate the damaging effects of high and low temperature on battery-cycle life.
The second approach uses science and technology to develop active materials that help boost cell energy, specifically energy density (Wh/L) and specific energy (Wh/kg). When combined with optimized electrolyte solutions, new cathode and anode materials with greater energy can significantly affect overall battery cost and range. Amplified energy means a smaller battery with less active and inactive materials for a much lower cost per unit of energy.
Three main materials influence cell energy: the anode, cathode, and electrolyte. Each is undergoing extensive research, and results so far promise to vastly improve Li-ion battery performance for applications in multiple arenas (see "Lithium-Battery Technology Drivers") .
Cathodes
Lithium cobalt oxide (LiCoO2) has been the industry-standard cathode material in Li-ion batteries for consumer electronics applications. The material offers excellent first-cycle efficiency surpassing 95%, and the ability to pack high densities (<20% electrode porosity) into the coated cathode electrodes for maximized energy cells. Over the last decade, advances in these materials have pushed them to higher voltages to further boost energy.
This file type includes high resolution graphics and schematics when applicable.
However, LiCoO2 cathode material is hampered by two drawbacks—it’s expensive due to the high cost of cobalt, and can be thermally unstable at elevated temperatures. Thus, a multitude of substitute cathode chemistries have emerged, such as lithium iron phosphate (LFP), manganese spinel (LMO), nickel cobalt aluminum (NCA), and mixed oxides of nickel, manganese, and cobalt (NMC).
Each chemistry has pros and cons. LFP offers high safety and cycle life, but less energy. LMO costs less, but energy is lower and it degrades in performance at high temperatures. Oxides of NMC show the most promise—they have a wide range of compositions and can be designed to offer a good blend of cost, energy, and safety. Blends of these cathode materials also have been employed to leverage strengths and minimize each chemistry’s limitations.
NMC-based oxides have proven to be a strong alternative because of their lower cost, solid energy density, and higher safety compared to LiCoO2, making them especially useful in electric vehicles since cost is a key driver for the selection of a cathode material. The cathode is the largest component of the battery’s raw material cost, and the cathode’s largest component cost is the base material.
Also important is the cost of processing cathode materials. A common approach to cutting cost is to scale back on the amount of cobalt used in compositions of NMC oxides. Cathode materials with fewer processing steps and lower-cost ambient processing conditions also can reduce cathode-material costs. It is best avoid cathode materials that require special handling in the battery-manufacturing process, because they will ultimately increase costs.
Anodes
Graphite, both synthetic and natural, continues to be the anode material of choice in Li-ion batteries. Over the last decade, battery manufacturers globally have optimized the battery-cell design by reducing the inactive materials via thinner current collectors (Cu for anode and Al for cathode) and thinner separators.
This approach led to incremental increases in the cell specific energy without changing the anode materials. However, the cell specific energy is approaching a level that requires new higher-energy anode materials in order to reach the next-step energy increment.
Silicon (Si) has emerged as an attractive choice because of its abundant supply, very low raw material cost, and ability to store large amounts of lithium. Using silicon as the anode also brings challenges, though, such as the need to increase first-cycle efficiency, improve the cycle life, and decrease the continuous expansion of electrodes.
Globally, there’s a great deal of research underway focusing on approaches to develop silicon anodes for Li-ion batteries. Silicon nanowires offer lots of energy, but the high surface areas lead to more reactions with the electrolyte and a drop in long-term performance. Processing issues may also emerge during the cell assembly.
Silicon-oxide type materials are available, too. However, their poor first-cycle efficiency (only about 75%) limits increases in cell energy (Wh/L), especially when compared to graphite-type material. Small increments in cell Wh/L are evident, but there’s no significant increase in cell Wh/kg. Silicon-carbon composites are also being studied, but the particle integrity under long-term performance is questionable.
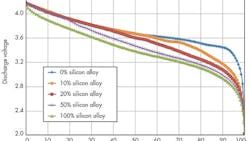
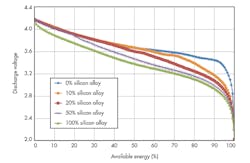
Silicon-alloy-type materials offer good particle morphology (optimized particle size, low surface area) along with higher first-cycle efficiency, resulting in higher-energy cells (both Wh/L and Wh/kg).
Researchers at 3M have invested in the silicon-alloy anode approach. The company has developed silicon alloys that are commercially viable, which lead to greater cell Wh/L and Wh/kg. These silicon alloys utilize water-based formulations, and are scalable in battery makers’ coating lines.
The company demonstrated the ability to fabricate these silicon-alloy anodes in commercially relevant quantities. Moreover, it improved performance by demonstrating commercial feasibility with greater than 70% capacity retention and less than 5% change in cell volume after 1000 cycles with 100% depth of discharge.
Electrolytes and Additives
Electrolytes and additives are critical to battery performance. Electrolytes help form a solid electrolyte interphase (SEI) between the active particles and the liquid electrolyte. A stable SEI layer passivates parasitic reactions, which is the key to a battery’s long life and cycle performance.
Each commercial cell maker has its own proprietary additives. Electrolytes and additives need to be developed with an optimized formulation for each combination of anode and cathode material. They also need a stable voltage window for optimum performance. Because electrolytes are flammable, the formulation can also contribute to battery safety in case of a thermal runaway.
Over the years, the industry has developed additives that can help reduce safety concerns. They are one of the key enablers in the performance of next-generation, high-voltage cathode materials and advanced alloy anodes. Additives come in many forms, but the use of fluorinated materials is very common due to their excellent electrochemical stability and very low flammability.
Succeeding at Synergy
Over the years, LiCoO2 matched to graphite has been the staple for portable consumer electronics markets. However, cell designers are reaching a point where active anode and cathode materials become limiting factors for enhancing energy density.
As mentioned earlier, LiCoO2 offers higher reversibility and significantly higher density in coated electrodes. It has evolved in performance and is difficult to beat for advanced consumer electronics applications. But in today’s high-tech mobile handheld devices, space is at a premium. One of the roadblocks to attain ever-thinner devices is battery thickness. That means, at least in the near future, greater use of novel higher-energy active materials.
Silicon-alloy anode materials developed by 3M help boost energy density (Fig. 1) and provide proper structure (amorphous active phase), particle morphology, and surface chemistry leading to controlled volume expansion upon lithiation and good cycling. Significant progress is being made to control cell expansion during cycling by developing the alloy composition and binder, optimizing the particle morphology, and optimizing the electrode composite.
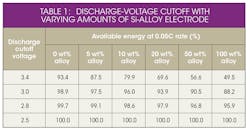
To obtain the maximum energy from a silicon-alloy anode containing a full cell, the lower cell cutoff voltage must be decreased (Fig. 2 and Table 1).
In terms of battery electric vehicles (BEVs), the cost of the battery pack (dollars per kilowatt-hour) has become the key challenge to their widespread adoption. To have a transformational change in the cell energy from the current commercial cell chemistry, materials with higher energy and better matched irreversible capacity must be developed. Also, it’s essential that the performance of these materials be validated in actual test vehicles during the materials development process.
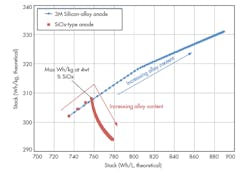
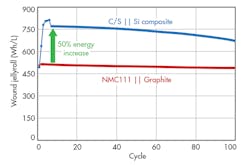
In a research project funded by the U.S. Department of Energy, 3M led a team of global companies and national laboratories with cross-functional expertise to target United States Advanced Battery Consortium (USABC) goals specific to the electric vehicle. Using a core shell cathode and a silicon-alloy anode, 3M achieved a 50% energy improvement over an NMC/graphite design (Fig 3).
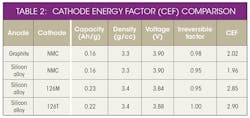
This material, supported by the Department of Energy under Award Number(s) De-EE0006448,* is based on a mixed NMC (low cobalt levels) oxide with a high nickel core and high manganese shell. The core shell (C/S) high-energy cathode technology developed by 3M exhibits a cathode energy factor (CEF) 35% higher than NMC 111 (Table 2).
New cathode materials with a CEF beyond the traditional LCO and NMC materials invariably require charging to higher cell voltage. To maximize the system’s energy, the cathode irreversible capacity should also “match” the irreversible capacity of the composite alloy anode.
In addition, for cell balance and control of lower cutoff cell voltage, the irreversible capacity of the composite cathode should be slightly larger than that of the anode. The electrolyte and separator must be designed to be stable against the two active composite electrodes, across the complete cell voltage range, to mitigate any parasitic reactions.
Figure 3 shows the performance of this synergistic system with a C/S cathode and silicon-alloy anode. The data, derived from an 18650-format cell, indicates a 50% energy improvement. The wet laminate energy excludes the package dimensions. This system is required in higher charge voltages (> 4.55 V) to obtain the maximum energy, although higher voltage cycling leads to some cycle-life challenges due primarily to electrolyte oxidation.
Cycle-life improvements were made during the development and testing of this unique synergistic concept. Post-mortem analysis of cells that lost nearly all of their capacity shows signs of electrolyte dry-out. However, the anode and cathode active materials exhibit nearly the same capacity as pristine active materials.
This suggests that a stable high-voltage electrolyte and additives system is one of the key parameters for long-life battery performance. Research is being conducted not only at 3M, but also in other top-tier global labs. The company is leveraging its expertise in electrochemical fluorination and materials synthesis to improve electrolyte performance.
This file type includes high resolution graphics and schematics when applicable.
*Disclaimer: This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Jagat D. Singh, senior product development engineer at 3M Company, holds an MS in materials science from the University of Central Florida and a BS in mechanical engineering from the University of Pune, India. He also is the principal investigator of two Department of Energy grants to develop and integrate advanced anode and cathode materials for batteries in vehicle electrification.