Hybrid Beta-Particle Battery May Produce Long-Term Power Without Recharging
What you’ll learn:
- How beta particles can be used to generate power.
- The challenges of building a cell based on beta particles.
- How some of these limitations were overcome.
- The remaining harsh realities of cells based on beta particles.
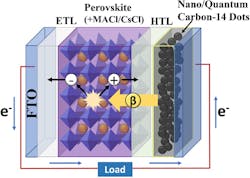
Researchers at the Daegu Gyeongbuk Institute of Science & Technology (DGIST), Republic of Korea, have achieved what they claim is a significant breakthrough by creating the world’s first next-generation “betavoltaic” cell. This advanced power source was made by directly connecting a radioactive isotope’s electrode emitting beta particles to a perovskite absorber layer, a cutting-edge material known for its efficiency. In theory, this harvesting could lead to long-life, pocket-sized power sources.
Perovskite, a calcium-titanium-oxide mineral, has been known since the early 1800s. Its name is also applied to the class of compounds that have the same type of crystal structure as CaTiO3, known as the perovskite structure, and has a general chemical formula ABX3 where A and B are cations and X is an anion, often oxides or halides.
Perovskites offer advantages such as high power-conversion efficiency, tunable bandgaps, and potentially lower production costs compared to traditional silicon solar cells. However, they also face challenges related to stability and long-term performance. As with graphene and other intriguing materials, there’s lots of investigative research being done on diverse and non-obvious applications for perovskites.
What’s a Betavoltaic Cell?
Betavoltaic cells generate electricity by capturing beta (β) particles emitted during the natural radioactive decay. Betavoltaic devices have a radioactive isotope as the energy source, a β-radiation absorbing material for energy conversion, and a counter electrode. (Note that this betavoltaic principle is not the same as using the long-lasting heat of radioactive decay and thermocouples in thermoelectric generators, or TEGs.)
The β-radiation-absorbing material plays a critical role in converting radiation energy into electrical energy, directly influencing the cell’s efficiency and stability. The energy conversion efficiency (ECE) of these devices is currently below 4%, and they have a maximum output power under 500 mW.
In theory, they can operate for decades without maintenance. Beta particles also offer some important biological-safety advantages, as they can’t penetrate human skin. Nevertheless, practical progress has been limited due to the challenges of handling radioactive materials and ensuring material stability.
A New Strategy to Boost Energy Conversion Efficiency
To enhance β-radiation absorption, new material strategies are needed. To boost performance, the DGIST team embedded carbon-14-based quantum dots into the electrode and improved the structure of the perovskite layer. These innovations led to a highly stable power output and relatively impressive ECE (Fig. 1); the key word here is “relatively.”
The interaction between β electrons and the heavy atoms (such as bromine, iodine, and lead) found in perovskites enhances performance by shortening the penetration depth of β particles, thus allowing for more efficient use of the β-radiation energy. In addition, perovskites demonstrate impressive radiation resistance.
Despite these advantages, these cells face several challenges related to achieving high efficiency and long-term stability. Their sensitivity to moisture and oxygen leads to degradation and phase transitions that compromise efficiency and long-term stability. In addition, factors such as the presence of defects, trap states, and the thickness of the perovskite film can degrade their ECE due to nonradiative recombination losses along with self-absorption and shielding effects.
The team’s strategy involved using methylammonium chloride (MACl) as an additive, which has demonstrated effectiveness in improving perovskite efficiency. It creates large perovskite domains, leading to large single-crystal grains with fewer defects and higher efficiency. However, the volatile nature of MACl poses stability concerns, as the methylammonium (MA) cation can cause degradation over time. To counter this, cesium chloride (CsCl) was suggested as a potential candidate for improving the long-term stability of the perovskite.
Testing for ECE Performance and Stability
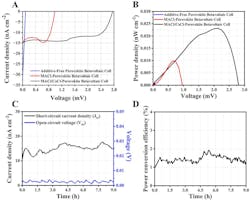
The team performed a full array of electrical, chemical, radiation, and stability tests on their device for factors such as open-circuit voltage (Voc), short-circuit current density (Jsc), maximum power density (Pmax), and ECE (Fig. 2). Their device was able to sustain an ECE of approximately 1.8 ± 0.2% for up to nine hours of continuous operation, which they maintain is a promising indicator of the device’s operational stability.
During this time, the device also maintained a stable power-conversion efficiency (PCE) of approximately 1.5 ± 0.2%. The cell demonstrated power density per radioactive source at 5.32 nW/cm2, Jsc of 15.01 nA/cm2, and Voc of 2.75 mV.
Looking at these numbers, they believe that the perovskite-based betavoltaic device has the potential to achieve the expected efficiency of 28%, comparable to that of a solar cell. As this is the first report of a betavoltaic cell directly integrated with perovskite, there remains substantial room for improvement.
Although it’s very unlikely you’ll see a practical, consumer-friendly, pocket-sized betavoltaic-based “power bank” soon due to efficiency issues and the “radioactive” aspects, it’s still an interesting exploration in alternative sources of power and unusual forms of energy harvesting. Their work is detailed in the paper “Novel perovskite-based betavoltaic cell: dual additive strategy for enhanced FAPbI3 α-phase stability and performance” published in Chemical Communications (Royal Society of Chemistry) along with a lengthy, highly detailed Supporting Information file.
References
Perovskite-info/Metalgrass, “An introduction to Perovskites.”
U.S. Department of Energy, Perovskite Solar Cells.
About the Author

Bill Schweber
Contributing Editor
Bill Schweber is an electronics engineer who has written three textbooks on electronic communications systems, as well as hundreds of technical articles, opinion columns, and product features. In past roles, he worked as a technical website manager for multiple topic-specific sites for EE Times, as well as both the Executive Editor and Analog Editor at EDN.
At Analog Devices Inc., Bill was in marketing communications (public relations). As a result, he has been on both sides of the technical PR function, presenting company products, stories, and messages to the media and also as the recipient of these.
Prior to the MarCom role at Analog, Bill was associate editor of their respected technical journal and worked in their product marketing and applications engineering groups. Before those roles, he was at Instron Corp., doing hands-on analog- and power-circuit design and systems integration for materials-testing machine controls.
Bill has an MSEE (Univ. of Mass) and BSEE (Columbia Univ.), is a Registered Professional Engineer, and holds an Advanced Class amateur radio license. He has also planned, written, and presented online courses on a variety of engineering topics, including MOSFET basics, ADC selection, and driving LEDs.