Nanostructures improve Li-ion batteries
Normal0falsefalsefalseEN-USX-NONEX-NONEMicrosoftInternetExplorer4
A nanomaterial developed at Rensselaer Polytechnic Institute could make lithium-ion batteries suitable for electric automobiles, as well laptops , mobile phones, and other portable devices. The material, dubbed a “nanoscoop” because its resembles an ice cream cone, withstands fast charges and discharges that would deteriorate electrodes on today’s li-ion batteries. In tests, the nannoscoop electrode could be charged and discharged 40 to 60 times faster than conventional battery anodes, while maintaining a comparable energy density. And the electrode survived 100 continuous charge/discharge cycles.
The anode of a li-ion battery expands and shrinks as the battery charges or discharges. These changes build up of stress in the anode which, if too rapid, lead to premature battery failure. That’s why most batteries in portable electronics intentionally charge slowly.
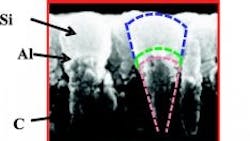
A nanoscoop consists of a carbon nanorod base topped with a thin layer of aluminum (Al) and a “scoop” of silicon (Si), making it flexible and able to quickly accept and discharge li ions .
For more information, check out this RPI press release.