Directed Light Shrinks Li-ion Battery Recharge Time
With so much attention on reducing the recharge time of lithium-based batteries, researchers are exploring novel, if perhaps impractical, approaches and “accelerants.” It’s not a matter of how much current the charging source can deliver, but how fast the chemical reaction within the cell that constitutes recharging can occur. Researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory have tested a scheme in which light is directed at the cell while charging—and it cuts the charge time by about half.
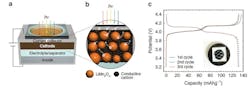
The normal charging reaction involves removing lithium from the oxide cathode and inserting it into the graphite anode. To explore the light-boosted charge process, the research team crafted small lithium-ion cells—commonly referred to as “coin cells”—with transparent quartz windows and then tested these cells with and without white light shining onto the cathode through the window (Fig. 1).
The cathode material was lithium manganese oxide, or LiMn2O4 (abbreviated as LMO). While absorbing the photons in the light during charging, the elemental manganese in the LMO changes its charge state from trivalent to tetravalent (Mn3+ to Mn4+). In response, lithium ions eject faster from the cathode than would otherwise occur without the photon-excitation process.
In formal terms, such an enhancement is enabled by the induction of a microsecond long-lived charge separated state, consisting of Mn4+ (hole) plus electron. It results in more oxidized metal centers as ejected lithium ions are created under light and with voltage bias. This situation, in turn, accelerates the battery reaction.
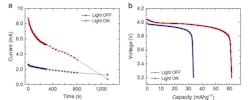
The “light-on” experiments were carried out under 1 SUN (100 mW/cm2) intensity. The Argonne team found that the faster reaction resulted in faster charging but without degrading battery performance or cycle life—important considerations in any recharge-acceleration scheme (Fig. 2). They used extremely sophisticated instrumentation and measurement techniques (after all, this is Argonne National Laboratory) to assess not only the cell’s performance and electrochemistry changes, but also any undesired side effects of the exposure to the Xenon lamp with UV filter, going far beyond standard voltage, current, and capacity metrics.
The cell exhibited normal function after the light test and Raman spectroscopy of the LMO material suggested no phase changes nor electrolyte decomposition/change. They also ran control tests to judge the detrimental effects, if any, of heat associated with the impinging light, but found these were negligible.
Of course, the unaddressed and thus unanswered question is the practicality, viability, and cost of “transparent” battery housings, and the physical challenges of shining an intense light on the battery cell or pack. The research team performed this work as part of the Center for Electrochemical Energy Science (CEES), a DOE Energy Frontier Research Center (EFRC) led by Argonne. The details of the electrochemical arrangement and analysis are intense and fully described in their paper “Photo-accelerated fast charging of lithium-ion batteries” published in Nature, with additional Supplementary Material available online.