Preventing Li-Ion Thermal Runaway During Battery-Cell Test
Much research and development today is spent trying to make lithium-ion cells safer. Some chemistries are inherently safer than others. For example, lithium-iron-phosphate (LFP) cells use chemically stable LFP, which doesn’t exhibit the energetic thermal runaway experienced by metal-oxide lithium-ion cells.
However, one must always be careful when handling and processing cells. When testing cells, the goal of the test may be to evaluate cell performance under stressful conditions, such as extremes of temperature or high charge/discharge rates. During such stressful tests, care must be taken to monitor for signs of the start of thermal runaway and then proceed with appropriate action.
What is Thermal Runaway?
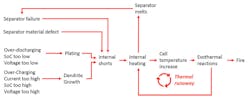
First, what is thermal runaway? Thermal runaway in a cell occurs when the cell internal temperature gets high enough to ignite the electrolyte, which is an organic liquid. Once the electrolyte is ignited, the oxide material in the cathode will break down and release oxygen. Now, in the damaged cell you have fuel (liquid organic electrolyte) and oxygen (from the oxides in the cathode)—ingredients for a fire that can generate its own oxygen, making it extremely difficult to extinguish (see figure).
For thermal runaway to start, enough heat must be generated in the cell to ignite the electrolyte. Normally, this happens because of damaged separator material. The thin poly-plastic material can be damaged by improper manufacturing or being punctured by metallic dendrite growth.
If the separator is punctured by a dendrite, the dendrite shorts the electrodes together. Current can freely flow as the anode and cathode come in contact through a short circuit where the damaged separator no longer keeps them apart. As current flows, heat is generated in the internal short and thermal runaway begins. More heat means more separator damage as the separator melts. More damage means more internal shorts, and more shorts mean more heat. Eventually, this heat can grow to the point where it ignites the electrolyte. The heat also causes the breakdown of the oxides in the cathode, which releases oxygen to feed the fire of the burning electrolyte.
Ending Thermal Runaway
Safety systems can be installed in laboratories or in manufacturing, basically wherever there’s the possibility for thermal runaway to start. Safety systems include gas/oxygen deprivation and water. Among the more common gas systems are carbon dioxide, argon, or nitrogen.
With these systems, the idea is to flood the area with gas that doesn’t contain oxygen and, therefore, makes it difficult for anything to burn. The problem is that since the damaged cell will generate its own oxygen, these systems will not put out the cell fire. The gas systems may prevent the spread of fire to nearby flammable materials (wires, shelves, plastic storage containers, etc.).
Water, on the other hand, works differently. Instead of trying to deny oxygen to the fire, water is applied to the cell to drop the cell temperature. The goal is to remove the heat to ensure electrolyte doesn’t burn as fuel and further oxygen isn’t released. Of course, a side benefit of water is that it will also stop nearby materials from burning. So, water is commonly used, even though it creates quite a mess as it’s sprayed onto a burning test setup.
Preventing Thermal Runaway
The best way to manage thermal runaway is to prevent it from starting.
First, cells should be manufactured with high-quality materials and with carefully controlled assembly processes to avoid separator defects. Ensuring proper alignment of electrodes and separators will prevent shorts around the edges of the stack (in pouch cells) or jellyroll (in prismatic and cylindrical cells).
During charging or discharging, it’s critical to ensure that cells aren’t overcharged or over-discharged, which can cause plating and internal shorts. By continuously monitoring cell open-circuit voltage, this will ensure cells remain within their safe operating range. By continuously monitoring the amp-hours of energy put into the cell, it will ensure too much charge isn’t pushed into the cell, which will cause overcharging.
These electrical limits are easily managed by properly designed cell test equipment that can immediately stop charging or discharging the cell if it detects electrical parameters that are incorrect. Such parameters include voltage too high, voltage too low, too much energy (amp-hours) pumped into the cell, or broken leads, which can cause measurement errors that obscure accurate detection of overcharging or over-discharging. For additional information about prevention of overcharging of cells, check out the article “Prevent Overcharging of Li-Ion Cells.”
Also during charging or discharging, cell temperature should be monitored. It’s to be expected that cell temperatures will rise during high-rate charging and discharging. When these high-rate charge/discharge steps are applied during elevated environmental temperature, it may be difficult to detect that the cell is getting too hot until it’s too late. Setting a proper thermal threshold would allow an alarm signal to be tripped and appropriate action taken before the onset of thermal runaway.
Once the thermal limit is tripped, that appropriate action should include immediate termination of all electrical activity; i.e., immediately stop charging or discharging the cells. Even after applied current is halted, the temperature of the cells should continue being monitored. If temperature continues to rise, the cells should be moved immediately to a water tank to cool down the cells. Alternatively, a sprinkler could be activated to douse the cells to lower their temperature.
These actions will keep the cell heat low enough to prevent separator melting, electrolyte ignition, and oxygen release from the cathode’s oxide coating.
Bob Zollo is the Solution Architect for Battery Testing, Electronic Industrial Solutions Group, at Keysight Technologies.