Download this article in PDF format.
From cell phones to electric vehicles, every user is concerned about runtime. System designers work diligently to maximize runtime using one of two approaches: design the battery-powered system to consume electricity efficiently so the batteries last longer, or maximize the amount of energy available to the battery-powered system. To maximize available battery power, you can use a larger battery or a high-capacity smaller battery. Since most battery-powered systems are portable, weight and size are considerations. As such, using a larger battery somewhat defeats the goal of smaller and lighter.
So, when building a battery, you’re best served by building a battery with high capacity. A battery is built up from cells, placed in series to increase available voltage and in parallel to increase available current. Thus, high-capacity batteries are built up from high-capacity cells. Today, the lithium-ion cell is the go-to cell for most battery-powered applications, with a great balance of size, weight, available current, capacity, and cost.
The Capacity of a Lithium-Ion Cell
Lithium-ion cells, or any cell for that matter, have a capacity measured in ampere-hours (Ah). For review, one ampere-hour means that you can draw one ampere from the cell for one hour. So, ampere-hours is the product of amperes times hours. Likewise, 1 Ah also means you can draw 2 A for 0.5 hours, or 0.25 A for four hours.
Ah capacity is, in fact, a measure of stored coulombs. Looking at units involved in ampere-hours, one ampere is 1 coulomb per second. If you multiply amperes × time, you get coulombs. Given that one hour is 3600 seconds, then 1 Ah is 3600 ampere-seconds, or (3600 coulombs/second) × seconds, which equals 3600 coulombs of stored charge in the cell. Note that for smaller cells, you may find their capacity measured in milliamp-hours, (mAh). For example, a typical 18650 lithium-ion cell will store around 3 Ah, or 3000 mAh.
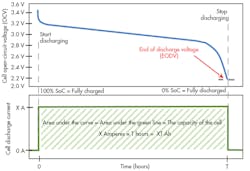
1. The illustration shows the discharge profile of a lithium-ion cell. The top line is the voltage vs. time, starting at fully charged and continuing until the end-of-discharge voltage (EODV) is reached. The current is constant during this discharge. The time measured is the length of time required to discharge. The capacity of the cell is the area under the discharge curve.
You can also measure cell capacity in watt-hours (Wh). Wh capacity is a measure of stored energy. Looking at units, one watt is one joule per second. If you multiply watts × time, you get joules. Given that one hour is 3600 seconds, then 1 Wh is 3600 watt-seconds, or (3600 joules/second) × seconds, which equals 3600 joules of stored energy in the cell.
The typical way, though, to describe the capacity of lithium-ion cells is their charge capacity, or Ah. For the remainder of this article, I will cover capacity exclusively in Ah.
To measure Ah capacity, start with a fully charged cell. The most basic way to measure the cell’s capacity is to draw a constant current of X amperes until it is discharged. The cell is considered discharged when the cell’s voltage reaches the end of discharge voltage (EODV).
To make a practical measurement, simply apply a fixed constant-current load of X amperes and start a clock. To be certain of the current being drawn, don’t rely on the constant-current load’s setpoint accuracy. Instead, measure the current that is being drawn by the load. We’ll call that measured current X amperes. Continuously measure the voltage on the cell. When the voltage reaches the EODV, stop the clock. Let’s say that is T hours (Fig. 1).
Now, simply multiply the constant-current value of X amperes × the measured time T. The result will be a measured capacity of X × T Ah. The capacity is the area under the current vs. time curve. In this simple measurement setup, the current vs. time curve is not a curve, but in fact a straight line. Therefore, the calculation of the area under the curve is simply X × T.
Factors Affecting Capacity Measurement Accuracy
In the above example, we were measuring three parameters: current, time, and voltage. Measuring time can be done with extreme accuracy, so the error in time measurement isn’t likely to a seriously negative impact on the capacity measurement.
Voltage measurement accuracy is important, because the ability to measure voltage is what stops the clock. If the voltage measurement is poor, it may stop the clock too soon, which would yield a capacity measurement that’s understated. Similarly, a poor voltage measurement can cause the clock to be stopped too late, yielding an overstated capacity. The good news is that the cell voltage is changing slowly vs. time. Therefore, the voltage measurement error can be mitigated by using a longer DMM integration time to reduce noise that could interfere with a good voltage measurement. Since the voltage is changing slowly, it’s safe to use the longer integration time.
Current measurement accuracy is the dominant factor in determining the error in the Ah capacity measurement. Poor current measurement accuracy will mean poor measurement of Ah capacity. To get a firm understanding of the quality of the Ah capacity measurement, look at the specifications of the current measurement you’re making.
Determining Capacity Measurement Accuracy
When measuring capacity, there will be a capacity measurement error in the form of a gain term of % of the capacity measurement plus an offset term of mAh of error per hour of measurement.
2. The Keysight Advanced Power System (APS) is a family of dc power supplies with 24 models at 1000 W (top) and 2000 W (bottom). These power supplies can both supply power and act like a constant-current load, while offering very high current measurement accuracy. For more information, check out www.keysight.com/find/APS.
Let’s take an example of measuring capacity with a Keysight APS 1000W Power Supply, model N7950A, rated at 9 V and ±100 A (Fig. 2). This power supply is a two-quadrant supply, which means it can both source (positive current up to +100 A) and sink current (negative current of up to ‒100 A). This makes it an excellent instrument for charging and discharging cells.
When discharging a cell or sinking current, the N7950A acts like a constant-current electronic load (e-load), and thus it can be used to measure cell capacity using the method described above. Note: For the remainder of this article, I will refer to this two-quadrant power supply as an e-load, since we are using it as an e-load to discharge the cell to measure the cell capacity.
Now, continuing the example, we will measure the capacity of a large cell, where we can pull a constant current of 5 A. This large cell is a pouch-type cell used in electric vehicles, perhaps with a capacity of 10 Ah or higher (Fig. 3).
The N7950A’s current measurement accuracy specification is 0.05% + 3 mA in the 0- to 10-A range. Remember, earlier I said it didn’t matter to what constant-current level the current was set because we would use the current measurement to determine exactly what current is being drawn from the cell. The N7950A also has a time-base accuracy of 0.01%.
3. Large-format lithium-ion pouch cells have been developed for use in electric vehicles. Large pouch cells can have capacities from 10 Ah to 40 Ah and beyond. For comparison, typical 18650 cylindrical cells are shown in the upper right corner of the photo.
To determine the gain term of the capacity measurement error, we need the sum of the current measurement gain accuracy of 0.05% and the time-base accuracy of 0.01%. Hence, the capacity measurement gain term will be 0.06% of the capacity measurement. So, if we measure a capacity of 10 Ah, then the 0.06% gain term will result in (0.06% × 10 Ah) = 6 mAh of error.
Now, let’s look at the fixed term. The APS in the low range has 3 mA of offset error. This says that there will be 3 mA of error over the integration period. As a result, there would be 3 mAh of error for every hour of measurement. Translating this to an easier form to make calculation, this would be 0.833 μAh per every second of measurement.
So, putting it all together:
- The e-load has a current measurement accuracy of 0.05% + 3 mA.
- The e-load has a capacity measurement accuracy of 0.06% + 0.833 μAh/second
- We’re measuring a current of 10 A for 1 hour because takes 1 hour for the cell to reach its EODV, which “stops the clock” on the capacity measurement.
- This would be 10 Ah of capacity.
- The capacity error gain term would be 0.06% of 10 Ah, or 6 mAh.
- The capacity offset term would be 0.833 μAh/second for 3600 seconds = 3 mAh.
- The total capacity error would be 6 mAh + 3 mAh = 9 mAh of error on 10 Ah of capacity measurement made over the course of 1 hour.
About the Author
Bob Zollo
Solution Architect for Battery Testing, Electronic Industrial Solutions Group
Bob Zollo is solution architect for battery testing for energy and automotive solutions in the Electronic Industrial Solutions Group of Keysight Technologies. Bob has been with Keysight since 1984 and holds a degree in electrical engineering from Stevens Institute of Technology, Hoboken, N.J. He can be contacted at [email protected].