Efficient heating and cooling for EVs
It's impractical to keep the passenger compartment of an electric vehicle at comfortable temperatures using the same means as on a conventional car. After all, there's little waste heat available for heating, and compressors for A/C consume a lot of juice, even when driven with super-efficient motors.
So the Pacific Northwest National Lab has come up with a concept for EV heating and cooling that works without enlisting waste heat or running compressors. In fact, its only moving parts are a couple of valves.
Key to the PNNL scheme is an adsorption heat pump that moves refrigerant through a heat exchanger using molecular energy rather than via mechanical means. But most adsorption heat pumps aren't particularly efficient. So PNNL researchers have come up with a twist on the idea that radically boosts the heating and cooling efficiency.
To understand PNNL's work, dubbed the Electric-Powered Adsorption Heat Pump for Electric Vehicles project, it helps to first review the principle of operation for a conventional adsorption heat pump.
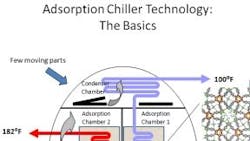
A standard adsorption chiller uses heat to boil refrigerant, usually water. Commercial adsorption units might use propane for a heat source, but waste heat and even solar power can serves as sources as well. The adsorptive material is usually silica gel. When the silica gel is dry, it has a great affinity for water. When a source heats up the adsorber, the high temperature induces a rise in pressure, from the evaporation pressure up to the condensation pressure. The cycle starts with the opening of a valve between the adsorptive silica material and the condenser. The adsorbent temperature continues to rise, which induces desorption of water vapor. The condenser liquifies the desorbed vapor. Meanwhile, the condensation heat is released at an intermediate temperature.
During cooling and depressurization, the adsorber releases heat while the valve is closed. The adsorbent temperature falls, which reduces the pressure from that of the condensation pressure down to the evaporation pressure.
During a cooling, adsorption and evaporation period, the adsorber continues releasing heat while being connected to the evaporator. The adsorber continues to cool, which lets it suck up vapor.
So essentially the process is one of a cooling mode where silica is absorbing refrigerant off the evaporator, then once the silica is saturated, begin applying heat to drive off as much of the water as possible, condense it, reject the heat outside, and repeat.
PNNL originally devised an adsoption system along these lines for use in buildings. But, explains PNNL Laboratory Fellow Peter McGrail, it had issues that made it impractical for use in EVs. Its efficiency, for example, was low because the silica jell has a relatively low capacity for sucking up water. And it takes quite a bit of heat to get the silica to give up water, so chillers have to be physically large.
Water also has high latent heat of vaporization, McGrail points out, and it is good for producing chilled water with a small volume. But the down side is that once vaporized, water has a low density. So it takes a lot of water vapor to get liquid water, and this drives up the size of the adsorption bed.
PNNL is trying to fix those issues by switching away from silica gel adsorbant material to a new class of advanced nanomaterial called an electrical metal organic framework (EMOF). EMOF material, says McGrail, can have triple and quadruple the mass uptake of refrigerant compared to silica gel. Its kinetics are also about a hundred times faster than that possible with silica gel, with about half of the adsorption penalty.
Some of the efficiency gains of this method come because the EMOF qualities would be controlled electrically, rather than by heating. PNNL hasn't built one yet, but computer simulation results have been promising, says McGrail. They show that the chiller can be about a factor of three smaller than when using a silica gel adsorbing material, with a coefficient of performance (COP) better by a factor of two. And that is without optimizing the heat transfer aspects of the system, McGrail says.
The only problem: PNNL has yet to fabricate an EMOF. To date, it only exists as a computer simulation. McGrail says the Lab is in the process of trying to make materials that exhibit the right qualities. "We are taking an organic compound that is conductive and marrying it with metals that are redox sensitive, that exist in different oxidation states," says McGrail. "We want to marry organic linkers with metal that can change its valence state, which would allow electrical current to move through the material. By changing the valence state of metals, we will change the binding affinity of the refrigerant."
PNNL is in the process of trying several different metals for use in EMOFs. McGail says by this time next year, researchers hope to find materials that can switch by electrical means and have the right amount of capacity to absorb a refrigerant. "By next year, we will know whether it will work. If we get that far, then we can move to the usual processes of refining it so the costs work out," says McGrail. If things work out, McGrail figures that process might start in about two years.
More info: http://arpa-e.energy.gov/ProgramsProjects/HEATS/ElectricPoweredAdsorptionHeatPumpforElectric.aspx
http://arpa-e.energy.gov/LinkClick.aspx?fileticket=HCoBAliXXWE%3d&tabid=525