“Fluid” Electrodes Enable Truly Flexible Batteries
What you’ll learn:
- How flexible, conformable electrodes can overcome product-packaging constraints imposed by their rigid counterparts.
- The soft-battery approach.
- The performance of batteries with flexible electrodes based on common materials.
The relative rigidity of batteries can be a problematic constraint when designing and packaging a product. This is especially the case for IoT-related applications such as wearables and IoT nodes, where the available space is limited and has an unusual shape or volume.
While advances have been made toward flexible or somewhat conformable batteries, their rigid electrodes still present packaging limitations. In existing stretchable battery designs, increasing the active material to yield higher capacity often leads to thicker and stiffer solid electrodes with poor mechanical properties
Electrode Goes from Solid to Fluid
Now, a team from the Researchers at the Laboratory of Organic Electronics at Linköping University (Sweden) has devised and tested an approach that transfers the physical property of a battery electrode from a conventional solid into a fluid state. The mechanical and electrochemical properties of their electrode rely on the viscosity of fluids rather than Young’s modulus of solids.
As the fluids conform easily into any shape with minimal force, they’re intrinsically deformable. Doing so decouples the electrochemical and mechanical property of the redox-active electrofluid, leading to higher capacities with more active material loading without stiffening the cell. Their cell showed excellent capacity retention over 500 charge-discharge cycles and mechanical robustness up to 100% strain.
Other attempts to develop soft and stretchable batteries have been based on different types of mechanical functions, such as rubbery composite materials that can be stretched out or connections that slide on each other. However, these don’t deal with the core of the problem: A large battery has higher capacity, but having more active materials means thicker electrodes and thus higher rigidity.
The team notes that fluid electrodes were tested in the past using liquid metals such as gallium, but with limited great success. However, these materials can only function as the anode and risk being solidified during charging and discharging, thus losing their fluid nature. In addition, many of the stretchable batteries previously made used rare materials that have a major environmental impact when mined and processed.
The Soft-Battery Approach
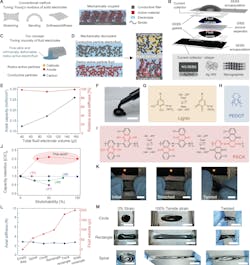
The team’s soft-battery approach is based instead on conductive plastics (called conjugated polymers) and lignin, a byproduct of paper production (Fig. 1).
The battery can be recharged and discharged over 500 times and will still maintain its performance. It can also be stretched to double the length and still work just as well (Fig. 2).
The material used offers a major advantage, noted Mohsen Mohammadi, postdoctoral fellow at LOE and one of the lead authors. “Since the materials in the battery are conjugated polymers and lignin, the raw materials are abundant. By repurposing a byproduct like lignin into a high-value commodity such as a battery material, we contribute to a more circular model. So, it’s a sustainable alternative.”
Performance of the Flexible Electrode
Many parameters are used to characterize the performance of a rechargeable battery. Among these are galvanostatic charge-discharge (GCD) curves, cyclic voltammetry (CV) curves, cell capacity under various conditions including strain (30, 50, and 100%), stretch cycling, and more. A few of these are shown in Figure 3.
The tests showed stable capacity retention and reversibility up to 100% strain and after 300 stretching cycles at 30% strain.
The team also acknowledges some shortcomings, with their next step being to increase the electrical voltage in the battery. According to team member Aiman Rahmanudin, “The battery isn’t perfect. We have shown that the concept works but the performance needs to be improved. The voltage is currently 0.9 V. So now we’ll look at using other chemical compounds to increase the voltage. One option that we are exploring is the use of zinc or manganese, two metals that are common in the Earth’s crust.”
The work is described in detail covering materials, fabrication, and tests in their paper “Make it flow from solid to liquid: Redox-active electrofluid for intrinsically stretchable batteries,” published in Science Advances.
About the Author

Bill Schweber
Contributing Editor
Bill Schweber is an electronics engineer who has written three textbooks on electronic communications systems, as well as hundreds of technical articles, opinion columns, and product features. In past roles, he worked as a technical website manager for multiple topic-specific sites for EE Times, as well as both the Executive Editor and Analog Editor at EDN.
At Analog Devices Inc., Bill was in marketing communications (public relations). As a result, he has been on both sides of the technical PR function, presenting company products, stories, and messages to the media and also as the recipient of these.
Prior to the MarCom role at Analog, Bill was associate editor of their respected technical journal and worked in their product marketing and applications engineering groups. Before those roles, he was at Instron Corp., doing hands-on analog- and power-circuit design and systems integration for materials-testing machine controls.
Bill has an MSEE (Univ. of Mass) and BSEE (Columbia Univ.), is a Registered Professional Engineer, and holds an Advanced Class amateur radio license. He has also planned, written, and presented online courses on a variety of engineering topics, including MOSFET basics, ADC selection, and driving LEDs.