DOE-Funded Consortium Aims to Reduce or Eliminate Critical Materials in Batteries
What you’ll learn:
- Sodium-ion battery chemistries have the potential to be lower-cost alternatives to lithium-based cells and could be produced in North America without the need to import lithium, carbon, cobalt, or other strategically sensitive materials.
- Na-ion cells face several obstacles to commercial success, including lower energy densities exhibited by most products presently under development.
- The LENS Consortium is already making breakthroughs that are advancing America’s leadership in the technology.
A strategic partnership between six of America’s leading national laboratories and eight academic partners is making good progress on developing commercially practical sodium-based batteries. Besides their potential to deliver significantly more kilowatt-hours per dollar, they could also end America’s dependence on imported lithium and other materials essential for the production the majority of today’s high-capacity batteries
A public-private collaboration known as the LENS Consortium (shorthand for Low-cost Earth-abundant Na-ion Storage) aims to overcome the technical obstacles involved with manufacturing sodium-ion batteries. The intent is to have Na-ion match — and eventually surpass — the energy density of lithium-iron-phosphate (LFP, LiFePO4) batteries. It would ultimately eliminate the need for lithium as well as the graphite, cobalt, and potentially nickel commonly used in the anodes and cathodes of lithium-based batteries.
To achieve this, each member of LENS is applying its own area of technical expertise to address the specific challenges associated with creating practical, long-lived elements of sodium-ion batteries. These include their anodes, cathodes, electrolytes, interfaces, inactive materials, and more.
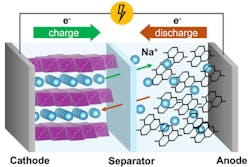
The figure below shows the working principle of sodium-ion batteries, whereby Na-ions migrate between a positive electrode (cathode) and a negative electrode (anode) through a sodium-ion electrolyte and a separator in between.
The LENS Consortium, led by Argonne National Laboratory, is funded by the DOE’s Vehicle Technologies Office. It includes six national laboratories: Argonne National Laboratory, Brookhaven National Laboratory, Lawrence Berkeley National Laboratory, Pacific Northwest National Labs (PNNL), Sandia National Laboratories, and SLAC National Accelerator Laboratory.
Participating universities are Florida State University, University of California, San Diego, University of Houston, University of Illinois Chicago, University of Maryland, University of Rhode Island, University of Wisconsin–Madison, and Virginia Tech.
PNNL Focusing on Advanced Electrolytes and Additives
In a recent press release, LENS partner PNNL announced that it’s developing a new class of advanced electrolytes and additives to enable long cycle life and safe operation for sodium-ion batteries.
“The first results are very promising,” said Phung Le, an electrochemist and PNNL’s principal investigator in the LENS consortium. “The electrolyte team has developed a localized high-concentration electrolyte, and we’ve shown that the electrolyte is compatible with high-voltage commercial pouch cells.”
Le also explained that the electrolyte appears to completely eliminate gas evolution during battery cycling. Such a phenomenon can cause sodium-ion batteries to swell, affecting performance and safety.
FSU's Mission to Boost Efficiency of Cathodes
In another development with a LENS partner, Florida State University’s research focuses on creation of more efficient cathodes, a critical component of every battery that determines how much energy can be stored in the battery and how much energy it supplies to a car. The FSU team seeks to overcome the lower energy densities available from today’s sodium chemistries caused by its weight, which is greater than lithium.
The FSU team hopes to overcome this disparity by creating new element and structure combinations to pack as much sodium in the cathode structure as possible. As a result, these new sodium-ion batteries would be even more powerful than lithium-ion.
Yan Zeng, FSU Assistant Professor of chemistry and biochemistry, explains, “We’ll use two different approaches to synthesize the target materials needed to create cathodes. The first is called solid-state reaction where we mix different element ingredients, like different metal oxides, carbonates, and other salts, and ‘bake’ them at a high temperature in chamber or tube furnaces.
“Our second approach is solution-based in which we crystallize a material from a solution, and the resulting materials are analyzed to determine what works together and what doesn’t. We have a robot arm doing repetitive work for the lab to increase efficiency, and we use AI algorithms to suggest what sorts of parameters could improve the outcome.”
Once the materials with these new elemental and structural combinations are created and analyzed, Zeng and her group will travel to national laboratories to collaborate on special designs enhancing the cathode alongside other scientists. Although the research underway by the LENS Consortium is only a year into its five-year program, it’s clear that the advances it’s making could be a game-changer for the North American EV industry.
“Sodium and lithium are very similar, and sodium batteries have been commercialized in other countries like China,” said Zeng. “However, there are no sodium-ion battery-powered electric vehicles in America yet — this is the country’s first big push toward environmentally friendly, affordable electric vehicles.”
Next in This Edition of PowerBites
More PowerBites
About the Author
Lee Goldberg
Contributing Editor
Lee Goldberg is a self-identified “Recovering Engineer,” Maker/Hacker, Green-Tech Maven, Aviator, Gadfly, and Geek Dad. He spent the first 18 years of his career helping design microprocessors, embedded systems, renewable energy applications, and the occasional interplanetary spacecraft. After trading his ‘scope and soldering iron for a keyboard and a second career as a tech journalist, he’s spent the next two decades at several print and online engineering publications.
Lee’s current focus is power electronics, especially the technologies involved with energy efficiency, energy management, and renewable energy. This dovetails with his coverage of sustainable technologies and various environmental and social issues within the engineering community that he began in 1996. Lee also covers 3D printers, open-source hardware, and other Maker/Hacker technologies.
Lee holds a BSEE in Electrical Engineering from Thomas Edison College, and participated in a colloquium on technology, society, and the environment at Goddard College’s Institute for Social Ecology. His book, “Green Electronics/Green Bottom Line - A Commonsense Guide To Environmentally Responsible Engineering and Management,” was published by Newnes Press.
Lee, his wife Catherine, and his daughter Anwyn currently reside in the outskirts of Princeton N.J., where they masquerade as a typical suburban family.
Lee also writes the regular PowerBites series.