The Temperature Effect: Tips When Testing Lithium-Ion Cells
Members can download this article in PDF format.
What you'll learn:
- The importance of keeping temperature constant in an environmental chamber.
- Proper test results means the cell's interior must be at a setpoint temperature.
- Issues with testing cells at room temperature.
When trying to characterize the behavior of lithium-ion cells, engineers and scientists know the temperature of the cell matters. Most notably, there’s a common understanding that cell reactions double for every 10°C, which is the Arrhenius equation.
For example, cell self-discharge is thought to double each time the cell temperature increases by 10°C. Given that temperature has such a significant impact, testing is done specifically under temperature control in environmental chambers. These chambers come in various internal volumes to accommodate cell samples from small sizes that can accommodate just a few cells to enormous walk-in chambers.
Constant Temp in Environmental Chambers
A key characteristic of the environmental chamber is the ability to hold its temperature constant to within a small variation, such as ±0.5°C or better. However, your environmental chamber may not achieve this performance. While the internal temperature, on average, can be held to a narrow band, conditions arise when this is not true.
Environmental chambers will cycle on/off to hold the temperature constant. This will create a cyclical temperature up/down pattern, on average, at the desired temperature.
In a large volume chamber, such as a walk-in chamber, there could be unevenness between regions within the chamber. As you fill a chamber to maximize utilization of the internal volume, airflow needed to homogenize the temperature within the chamber will be impeded by the large number of cells in the chamber, and hot or cold spots can develop.
Lastly, as you put cells into the chamber, the temperature of the freshly loaded cells most likely will not match the internal temperature of the chamber. This can drive the internal temperature off the setpoint. When that happens, the environmental chamber’s heating/cooling system will need to compensate for this temperature difference. It may take minutes or even hours for the chamber to recover and for the cells to reach the chamber’s setpoint.
Reaching Setpoint Temp Inside and Out
Once the temperature of the chamber is stabilized, does this mean the cell has reached the temperature of the chamber? The chamber is typically filled with air, which should be at the setpoint temperature. The cells are sitting in that air, so given enough time, the cell skin temperature will reach the chamber’s setpoint temperature. The temperature you can measure at that moment is either the chamber air temperature or the cell’s skin temperature using a surface-mounted temperature sensor.
However, the reactions that govern the cell behavior are happening throughout the volume inside of the cell. Until the temperature deep within the cell reaches the chamber’s setpoint temperature, there will be a mismatch between the cell behavior and the temperature you’re measuring.
How long does it take for the temperature to “soak into” the cell? Figure 1 shows an example. Understanding how long it takes for the cells to change temperature is critical whenever you’re running dynamic experiments (like temperature cycling) or when making sensitive measurements where small changes in cell temperature can impact the test results. An example of the latter case is measurement of cell self-discharge using the delta-OCV method. See this article for an explanation of how to measure self-discharge using the delta-OCV method.
Testing at Room Temperature
Not all testing is completed in an environmental chamber—it’s also performed on cells under room temperature with no direct temperature control. For example, during cell formation on a pilot line or large-scale manufacturing line, it may not be practical to control temperature using thermal chambers. Instead, the overall environment, such as the factory floor, is maintained at room temperature, typically 23°C ± 3°C.
As you can imagine, within the expanse of a factory held to this environmental specification, you will encounter hot spots and cool spots. This means as you move cells around inside the environment, as much as a 6°C difference may exist between the cells and their environment. When cells are brought into an area that’s different by a few degrees, the cells will need to reach thermal equilibrium with their new environment if you’re conducting measurements where temperature matters.
The thermal mass of the cells plays an important role in how quickly the cells achieve thermal equilibrium. A single 18650 cell may reach thermal equilibrium with the environment within a few minutes. However, if you put large prismatic cells into a high-mass metal pressure tray that can apply high pressure, you will have a large single thermal mass comprised of the tray and the cells themselves. This thermal mass will resist changing and can work to your advantage when temperature stability is required.
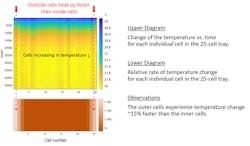
But, when sensitive measurements need to be made, even this large thermal mass may not offer sufficient stability. The edges of the mass will more quickly change temperature than the center of the mass. Figure 2 shows this effect.
In Figure 2, there’s a pressure tray that contains 25 prismatic cells. The cells are lined up in a single row and pressed together by end plates. As the environmental temperature changed by 2°C over the course of 12 hours, the individual cell temperatures were measured. The graphic shows how the end cells experienced a larger temperature change than the inner cells. These end cells were more exposed to the environment.
Even a Small Change Matters
Let’s look at an example of how this small change matters by going back to the delta-OCV measurement described earlier in this article. Remember, cell OCV is a function of temperature. A previous article describes how cell OCV changes with temperature.
When measuring changes in OCV, self-discharge and temperature will both contribute to the OCV change. Take, for example, the measurement of self-discharge using delta-OCV measurement on Figure 2’s example tray of 25 prismatic cells in a pressure tray. The temperature difference between the end cells and the middle cells is sizable enough that the cells on the end could have a measurably different OCV caused by the temperature change.
If you’re not accounting for that phenomenon, your delta-OCV measurement will misidentify cells on the end to possess a different self-discharge rate then cells in the middle. Of course, the difference in OCV isn’t caused by a difference in self-discharge rate. The difference in OCV is caused by a large variation in temperature experienced by the end cells relative to the center cells, even though it would seem the whole tray of cells is exposed to the same thermal conditions.
Temp-Management Tips
In summary, understanding how temperature impacts cells during test is critical during dynamic testing (temperature cycling) where temperature-stabilization time is a factor and when making sensitive measurements like delta-OCV for measuring self-discharge. Here are some tips to help manage the temperature effects:
- Take advantage of thermal mass: Even though individual cells may have relatively small thermal mass, a large group of cells and cells in high-mass metal trays will resist change in temperature. The cells and tray will stabilize small temperature changes.
- Insulation: Even when you have a large thermal mass, the cells on the ends and edges will heat up and cool down faster than the center of the mass. Whenever possible, use insulation around the tray to minimize this effect.
- Allow sufficient time for equilibration: When cells at stable temperatures are needed, make sure there’s enough time for the cells to reach thermal equilibrium in the environmental chamber. If you’re testing at room temperature, moving cells into the room where the testing will take place and queuing the cells near the tester will give additional time for cells to equilibrate with the environment without tying up the tester waiting for cells to stabilize.
- Characterize thermal behavior: Run specific experiments to understand how long it takes for cells to equilibrate based on your process steps. Knowledge is power. Once you understand this behavior, you can design your test process so that temperature equilibration is properly accounted for.
- Hold temperature constant: Whether you’re testing in an environmental chamber or out on the factory floor, if the cells are sensitive to temperature changes, keeping the cells at a constant temperature will facilitate faster testing with more reliable results.
Lastly, I’d like to thank my colleague Manuel Moertelmaier, Physicist and Researcher at Keysight Labs in Linz, Austria, who created the temperature/OCV model that provided valuable insights into temperature-related cell behavior. He also performed the analysis of temperature changes within the tray of cells.
About the Author
Bob Zollo
Solution Architect for Battery Testing, Electronic Industrial Solutions Group
Bob Zollo is solution architect for battery testing for energy and automotive solutions in the Electronic Industrial Solutions Group of Keysight Technologies. Bob has been with Keysight since 1984 and holds a degree in electrical engineering from Stevens Institute of Technology, Hoboken, N.J. He can be contacted at [email protected].