Understanding the Challenges of Measuring Battery Cell Voltage
Knowing the voltage of a battery cell is essential for ensuring the cell’s health, state of charge (SOC), safety, and performance. Monitoring cell voltage while the cell is in use can help identify problems such as wear-out, improper charging/discharging, or internal faults.
During testing, accurate cell voltage measurements are critical for evaluating how the cell responds under load, during charging and discharging cycles, and across various environmental conditions. This data helps identify issues such as capacity loss, cell imbalance, or potential safety risks like thermal runaway.
Inaccurate voltage readings can lead to incorrect test results, potentially masking defects or causing cells to be improperly characterized or graded.
Quality Voltage Measurements are Essential
Let’s look at factors that impact the quality of cell voltage measurement and how errors in these measurements can affect the quality of the test results.
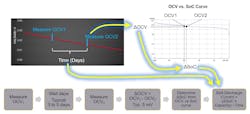
A special consideration for measuring cell voltage is needed when trying to measure the effect of cell self-discharge. This measurement is typically made by a method called Delta-OCV. Figure 1 explains this method, which involves measuring the OCV (open circuit voltage) of the cell twice and comparing the change (i.e., the delta) in OCV.
Thus, understanding the accuracy of the OCV measurement is doubly important when making Delta-OCV measurements.
DMM Error
Whether measuring OCV or Delta-OCV, the measurement error from the digital multimeter (DMM) will be a major consideration. Figure 2 compares the measurement error from two different DMM types. The left column represents the typical performance of the best DMMs available on the market. These will have 8.5 digits and are found in calibration labs.
As the table shows, when making a measurement on a cell, the measured voltage will be typically 4.2 V or less. For the example in the table, the cell voltage is 3.6 V. To measure 3.6 V, the DMM must be on a 10-V range. Results reveal the measurement error is ±15.3 μV when measuring the cell OCV.
The right column of the table represents the typical performance of a 6.5-digit DMM that commonly found on an engineer’s lab bench or integrated into a battery test system. As before, this DMM would need to be in the 10-V range, resulting in a total error of ±148 μV when measuring the cell OCV of 3.6 V. Since most cell measurements will be made with a 6.5-digit DMM, for the remainder of this article, I’ll use ±150 μV as the typical total error.
>>Download the PDF of this article
Figure 3 shows how to apply this DMM error to the measurement of cell OCV. The error of ±150 μV creates an error band of 300 μV, and the resultant error is 0.004 % of the 3.6-V cell OCV reading. It’s generally accepted by cell test engineers that a 0.004% reading is a good, high-quality reading.
A Deeper Dive into Delta-OCV
Now let’s look at the Delta-OCV measurement, where we’re not merely trying to obtain an accurate reading of the cell’s OCV. Instead, we’re trying to determine the difference between two OCV values. The typical Delta-OCV is 5 mV, so any total error will be referenced to the 5-mV difference, not the 3.6-V total OCV.
Figure 4 shows the calculation of error when making two OCV measurements. For Delta-OCV, the two errors bands of 150 μV need to be summed together because each OCV measurement will have an error band of 150 μV.
Furthermore, 300 μV of error isn’t on a 3.6 V measurement, but on a 5-mV voltage difference and thus represents a 6% error on that 5-mV measurement. This 6% error could be a surprising result if the Delta-OCV error was expected to be similar to OCV error.
The Impact of Cell Relaxation
Cell relaxation occurs following a change in SOC caused by charging or discharging. Cell relaxation is the process where the cell's voltage settles or stabilizes after being charged or discharged. After charging, the cell OCV will drop (relax downward), and after discharging, the cell OCV will rise (relax upward).
A cell’s OCV can change by several millivolts during the first few hours of relaxation. Therefore, measuring OCV or Delta-OCV before the cell is relaxed will give incorrect results.
The challenge is to know how long to wait for the cell to become fully relaxed. This needs to be characterized to be certain. The OCV change caused by relaxation will interfere with obtaining a correct OCV measurement. It could take 10 days for a cell to fully relax and have no change in OCV from relaxation.
Temperature Effect on Cell OCV
Cell OCV is a function of both SOC and temperature. While everyone understands that you can measure the cell’s OCV and estimate the SOC by looking at the OCV vs. SOC chart, understanding the impact of temperature effect is less commonly understood.
The OCV of the cell is determined, in part, by the cell’s SOC. However, the temperature of the cell is another important factor, as the cell’s OCV will vary with temperature. This effect is called the temperature coefficient of voltage (TCV) of the cell. It’s measured in microvolts per degree C (μV/°C).
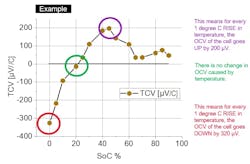
As shown in Figure 5, TCV can be positive, where an upward change in temperature leads to an upward change in OCV and a downward change in temperature leads to a downward change in OCV. Likewise, TCV can be negative, where an upward change in temperature leads to a downward change in OCV and a downward change in temperature leads to an upward change in OCV.
The polarity and size of the cell’s TCV is a function of the cell’s SoC (Fig. 5, again). For lithium-ion cells, a large positive TCV would be 200 μV/°C, while a large negative TCV would be −300 μV/°C.
For lithium-ion cells, the temperature impact can create up to a 300-μV change in OCV when there’s a 1°C change in cell temperature. If the cell R&D lab or cell factory temperature is ±2°C, the cell is exposed to a total temperature excursion of 4°C, which can cause up to 1.2 mV of OCV change.
Such an impact is much larger than DMM error when making a single OCV measurement. However, when measuring Delta-OCV with a 5-mV change, this 1.2-mV temperature-induced change is equivalent to a 30% error in Delta-OCV results.
Summary
Because cell voltage is a fundamental measurement, understanding the error sources in cell OCV measurements is critical. Key factors to keep in mind:
- When measuring a cell OCV of 4 V, a high-quality DMM will have approximately 150 μV of error, or a very low 0.004% error. However, when measuring Delta-OCV of 5 mV, the DMM error sums up to 300 μV, which is 6 % error on the 5-mV Delta-OCV value.
- Cell relaxation occurs following a change in SOC caused by charging or discharging. It results in several millivolts of change during first few hours of relaxation that must be accounted for when making decisions based on cell OCV measurements.
- Cell OCV is a function of temperature, and temperature impact can be up to 300 μV for 1°C change. Normal room temperature can easily change by ±2°C, causing up to 1.2 mV of OCV change that must be accounted for when making decisions based on cell OCV measurements.
Improperly accounting for these errors will cause cells to be incorrectly measured or improperly graded. It’s advisable to collaborate with measurement experts in the Test industry who can determine the true and traceable performance of your OCV measurements to help shorten aging and improve measurements.
>>Download the PDF of this article
About the Author
Bob Zollo
Solution Architect for Battery Testing, Electronic Industrial Solutions Group
Bob Zollo is solution architect for battery testing for energy and automotive solutions in the Electronic Industrial Solutions Group of Keysight Technologies. Bob has been with Keysight since 1984 and holds a degree in electrical engineering from Stevens Institute of Technology, Hoboken, N.J. He can be contacted at [email protected].