This article is part of the RoHS and Critical Materials in our Series Library.
This file type includes high-resolution graphics and schematics when applicable.
Gallium (Ga) is a unique metal used in making many types of electrical and electronic products perform efficiently. Though used in small amounts, it’s still a limited resource. You have no doubt heard “reduce, reuse, and recycle,” and over the past few decades, significant progress has been made in the recovery of electronics “end-of-life” products. While many of the metals used in electronic products have now achieved significant levels of recycling, such is not the case for minor metals like gallium.
Notable in their ability to improve speed and power-handling capability are gallium-based devices like GaAs and GaN (see "WBG Power Transistors Push the High-Power Envelope"). Besides semiconductors, Ga is used in other rapidly growing optical applications, including LEDs, displays, and fiber-optic networks (see "High-Speed Interfaces Push Optical Connectivity").
Ga is also a key element in photovoltaic (PV) cells based on copper indium gallium selenide (CIGS) and GaInP (see "Dual-Junction Solar Cell Breaks Efficiency Record"). These panels are much greater in area than microwave chips or LEDs, with proportionally greater material demand. And the deployment of PV cells continues to grow in the quest for clean, renewable energy.
Another sometimes overlooked application of Ga is in high-efficiency rare-earth (NdFeB) magnets, where small amounts of Ga may be added to optimize performance. Because magnets are more massive than microchips, and can be employed in large-scale energy-related applications like high-efficiency motors and wind-turbine generators, the ultimate Ga demand for these kinds of magnets could significantly add to the aforementioned applications.
Concerns about limited natural resources and energy, coupled with growing amounts of consumer end-of-life electronic product waste, have given rise to regulatory requirements and voluntary initiatives to improve product sustainability. Disciplines like Industrial Ecology have become the basis of a total lifecycle approach, where raw-material extraction, production, use, and end-of-life phase all must be considered.
Design for Environment methods for electronic and electromechanical products are also becoming standardized. While overall adoption of these best practices has been substantial, achieving lifecycle maturity for Ga, in particular, has yet to be realized. As it turns out, this is not easily accomplished and remains a long-term challenge.
Next we review the availability of Ga from naturally occurring sources and where it’s used. This quickly leads us to consider elements beyond Ga itself. We then discuss how basic principles of sustainability and product stewardship could be applied to achieve an improved Ga lifecycle, and conclude with some prospects to improve this metal’s sustainability.
Sources of Gallium
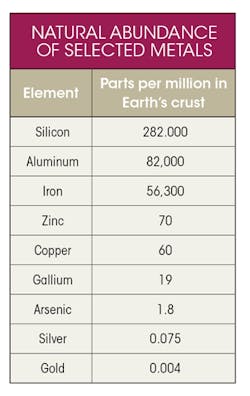
The overall availability of elements depends on several factors, including natural abundance; ores having sufficient concentration to enable economically viable extraction; commercially available processes for refining; and established flows to recycle waste. Gallium is relatively rare, and is not found naturally as the metal itself. It occurs mainly as the mineral gallite, CuGaS2. Although the copper (Cu) might suggest that copper would be a natural source of gallite, it is not found in significant concentrations in copper ore, instead is only concentrated enough for commercial extraction as a secondary product of aluminum and zinc.—a relatively rare substance on its own—
The table lists the naturally occurring global concentration of elements commonly used in electronics products. Gallium is not a “Rare Earth” metal, but it is, indeed, relatively rare.
The familiar element silicon, which is highly purified for use in microchips, is comparatively unlimited as a starting material, as are common mechanical and structural metals like aluminum and iron. It’s the relatively low but commercially viable concentration of gallite in bauxite, the naturally occurring form of Al, which we will find contributing to the sustainability of Ga. To summarize, Ga is a limited resource and is a child metal dependent on other metals for its production.
It’s worthy of note that Ga is actually less abundant than some of the 17 rare-earth elements, including cerium, lanthanum, neodymium, scandium, and yttrium (not shown in the table). The rare earths deserve special mention because we refer to them later in the context of energy-critical elements and their lifecycles and sustainability.
Gallium Recycling
Recycling of precious metals like silver and gold has existed for millennia due to their even more limited resource availability and highly desired qualities for a variety of monetary, jewelry, and technical applications. Aluminum, iron, zinc and copper, as well as other commodity metals, enjoy a robust infrastructure for recycling.
The main sources are so-called new scrap from manufacturing processes prior to being sold on the market, and as old scrap from items discarded at end-of-life of their intended use. Both of these sources, plus virgin ore, are then made into new metals to sustain their lifecycle and make new products. In addition to helping close the loop on material reuse, significant energy savings may result when processing recycled metals as compared to virgin ore.
GaAs can be recycled internally from new scrap generated in operations like wafer processing. But neither Ga or As have achieved commercial recycling of old scrap. Whether this is an immediate concern is discussed below.
Much can be learned from existing success in recycling the various materials in high-volume consumer products. Separation and recycling technologies exist to improve the closed-loop resource usage of ferrous and non-ferrous metals, precious metals, and to a lesser extent, glass, ceramics, and plastics.
While metals generally can be recycled and reprocessed virtually indefinitely into making new products, nonmetals and plastics tend to be downcycled into less-critical applications. In many regions, the major metals in high-volume consumer devices like cell phones can be dealt with in an environmentally sound manner, while rarer metals like Ga tend to end up more or less “unaccounted for” in what remains a waste fraction.
Opportunities for Ga Lifecycle Optimization
Materials flow-analysis techniques have shown that Ga is currently recycled only from new scrap in GaAs wafer manufacturing. How can Ga use become more sustainable? Solutions may require combined materials recovery, including uses of Ga beyond semiconductor chips.
Somewhat surprisingly, coal ash has been reported as a significant source of Ga. While we would not generally promote burning more coal for that purpose, existing piles of coal ash could be an interesting potential source of old Ga scrap.
Due to the energy-saving and energy-generating aspects of LEDs, photovoltaics, and rare-earth magnets, and their reliance not only on Ga but other scarce elements, focus has been brought to bear on assuring a sustainable supply of these elements. Recognition of these energy-critical elements is the first step in creating awareness and establishing policies that may help promote the sustainability of these elements as a general goal.
Product designers should strive to reduce materials use, minimize their variety in a product, and choose materials with lower environmental impact and those that are easily recycled. Unfortunately, these goals are difficult to realize, since Ga in microcircuits is actually used to replace—for performance reasons—the more abundant Si. On the positive side, minimal amounts of Ga are inherently used by design. The recycling of GaAs from new scrap (wafer manufacturing) has already been mentioned.
Emerging new materials like graphene may hold promise as an alternative material, since graphene is based on very abundant carbon. Another novel approach would substitute oxides of more common metals for those of limited supply. At this time, these are still areas for future development.
End-of-Life Product Issues
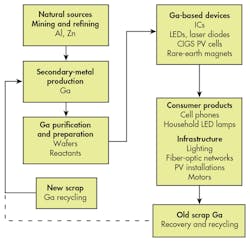
One of the means to improve recovery of any material is to ensure that it can be easily separated from other fractions to improve the purity of the recycling stream. It might be possible to use the natural grouping of GaAs containing devices on a particular area of a cell-phone circuit board, for example, but generally the entire board is considered a recyclable fraction by itself. Though the major metals mentioned so far can be recovered by smelting and refining, that’s not yet the case with trace elements like Ga. The figure summarizes the current lifecycle of Ga, showing that fully-closed-loop recovery has yet to be achieved for old scrap, i.e., end-of-life products.
Does Ga need an established old scrap recycling flow of its own? The answer depends on several factors, but as of now, the availability of primary sources, namely Al from bauxite, ensures a sufficient supply. This picture could change if demand for Ga grows significantly for current applications or new technologies.
Like any commodity, the future recovery of Ga will be driven by its concentration vs. price curve. A combined means of concentrating Ga from all end-product applications would then likely be the key to improved closed-loop recycling. Today, special collection and treatment of Ga-containing end-of-life products—PV installations, LED lighting, fiber optics, and rare-earth motors—remains to be established. Supply and demand will drive upcoming trends in this area.
Conclusion
We have summarized the available sources for Ga, its electrical and electronic applications, and the current state of closed-loop recovery. Improved recovery of Ga in the long term will depend on its demand and the ability to economically concentrate it from various sources for recycling. Presently, Ga slightly fails to reach a sufficient concentration in waste streams like mobile-phone circuit boards, PV installations, fiber-optic communications, and lighting applications, to fully close the loop. Collecting and combining Ga-containing products from several of these waste streams could change this scenario in the future.
Read more articles from the RoHS and Critical Materials in our Series Library.
Looking for parts? Go to SourceESB.
References:
R.U. Ayres and L.W Ayres, Industrial Ecology: Towards Closing the Materials Cycle, Cheltenham, UK: Edward Elgar, 1996.
ECMA-341 Standard, Environmental Design Considerations for ICT & CE Products, 4th Ed., Geneva, CH: ECMA International, December 2010.
International Standard IEC 62430: Environmentally Conscious Design of Electrical and Electronic Products and Systems, Geneva, CH: International Electrotechnical Commission, 2009.
W.M. Haynes and D.R. Lide, Eds., CRC Handbook of Chemistry and Physics, 92nd ed., New York: CRC Press, 2012.
B.W. Jaskula, 2013 Minerals Yearbook: Gallium, Reston, VA: U.S. Geological Survey, June, 2015.
R.L. Franz, “Optimizing Portable Product Recycling Through Reverse Supply Chain Technology,” IEEE Intl. Symp. Elec. Environ., pp. 274-279, May, 2002,
UL White Paper, “The Life Cycle of Materials in Mobile Phones,” accessed Sep. 3, 2015.
P.Y. Dehnavi, “Global Cycle of Gallium Production, Use and Potential Recycling,” TRITA-LWR Degree Project 13-23, Stockholm, SE: Royal Institute of Technology, 2013.
T. Uryu, J. Yoshinaga, and Y. Yanagisawa, “Environmental Fate of Gallium Arsenide Semiconductor Disposal: A Case Study of Mobile Phones,” J. Ind. Ecol. vol. 7, no.2, pp. 103-112, 2003.
A.N. Lovik, E. Restrepo, and D.B. Muller, “The Global Anthropogenic Gallium System: Determinants of Demand, Supply and Efficiency Improvements,” Environ. Sci. Technol., vol. 49, pp. 5704-5712, 2015.
The American Physical Society and the Materials Research Society, Energy Critical Elements: Securing Materials for Emerging Technologies, Washington, DC: APS, 2011.
P. Asbeck, K. Lee, and J-S. Moon, “Graphene: Status and Prospects as a Microwave Material,” IEEE 12th Annual Wireless and Microwave Technology Conference (WAMICON), 2011.
H. Hosono, K. Hayashi, T. Kamiya, T. Atou, and T. Susaki, “New functionalities in abundant element oxides: ubiquitous element strategy,” Sci. Technol. Adv. Mater. vol. 12, 034303 (22pp), 2011.
J. Johnson, E.Harper, R. Lifset, and T.E. Graedel, “Dining at the Periodic Table: Metals Concentrations as They Relate to Recycling,” Environ. Sci. Tech., vol. 41, no. 5, pp. 1759-65, 2007.
>> Electronic Design Resources
.. >> Library: Article Series
.. .. >> Topic: System Design
.. .. .. >> Series: RoHS and Critical Materials
About the Author
Roger L. Franz
Environmental Sustainability Advocate and Contributing Author
Roger Franz has served as Principal, Engineering IT at TE Connectivity, Research Engineer at UL Environment, and Group Leader and Design for Environment Manager at Motorola. In addition to product environmental compliance and standards, his passions include new product development, quality, and technology research. His papers have appeared in several industry journals. He has had over a dozen article published in Electronic Design. He holds a BA and MS in chemistry from Grinnell College and Northwestern University, respectively.